

二氟草酸硼酸钠作为钠离子电池非水电解液添加剂的电化学性能
收稿日期: 2016-07-03
修回日期: 2016-08-25
网络出版日期: 2016-11-02
基金资助
国家自然科学基金(No.21506141),山西省自然科学基金(No.2015021131, No.201601021040)资助项目
Electrochemical Performance of Sodium Difluoro(oxalato)borate as the Additive of Non-aqueous Electrolytes for Sodium-ion Batteries
Received date: 2016-07-03
Revised date: 2016-08-25
Online published: 2016-11-02
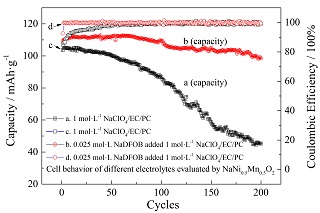
引入电解液添加剂是提升钠离子二次电池电化学性能的重要途径.本论文制备了二氟草酸硼酸钠(NaDFOB)并作为NaClO4/碳酸乙烯酯(EC)/碳酸丙烯酯(PC)( EC:PC体积比=1:1)非水电解液的添加剂,分别考察了其加入量对于电导率特性、电化学氧化分解电压的影响,以及应用于NaNi0.5Mn0.5O2半电池的电化学性能. 结果表明,NaDFOB作为添加剂时对于NaClO4/EC/PC电解液电导率提升不明显,但是显著提升了电解液的氧化分解电压;以添加0.025 mol·L-1 NaDFOB的电解液应用于NaNi0.5Mn0.5O2半电池时,首周不可逆比容量由22 mAh·g-1下降到9 mAh·g-1,同时0.2C倍率下循环200周容量保持率由44.4% 提升到89.5%,平均每周容量衰减为0.06 mAh·g-1. 因此,NaDFOB可以作为钠离子电池非水电解液的一种有效添加剂.
张鼎 , 朱芹 , 王瑛 , 赵成龙 , 刘世斌 , 徐守冬 . 二氟草酸硼酸钠作为钠离子电池非水电解液添加剂的电化学性能[J]. 电化学, 2017 , 23(4) : 473 -479 . DOI: 10.13208/j.electrochem.160703
Sodium ion battery has attracted worldwide and intensive attention recently, while the adoption of electrolyte additives has been considered as one effective strategy to promote the cell performance. Within this work sodium difluoro(oxalato)borate (NaDFOB) was prepared and adopted as an additive for the general non-aqueous electrolyte formula of 1 mol·L-1 NaClO4/EC/PC (Vol: Vol=1:1), and the effects of the additive concentration on ionic conductivity and oxidization decomposition voltage were investigated in detail. In addition, the cell performance evaluated by NaNi0.5Mn0.5O2 as the cathode was also studied. It reveals that the addition of NaDFOB into the NaClO4/EC/PC electrolyte resulted in the significantly increased oxidation decomposition voltages from 4.6 V to 4.85 V, in spite of the slightly increased ionic conductivity, attributed to extra dissociated sodium salt NaDFOB. When the 0.025 mol·L-1 NaDFOB added electrolyte was used to support the operation of NaNi0.5Mn0.5O2 cathode, the initial irreversible capacity decreased from 22 mAh·g-1 to 9 mAh·g-1, and the capacity retention upon 200 cycles at 0.2 C-rate increased from 44.4% to 89.5%, with an average capacity fade of 0.06 mAh·g-1 per cycle. Therefore, the NaDFOB was proved to be an effective electrolyte additive for non-aqueous sodium ion batteries.

/
| 〈 |
|
〉 |