

Pd/Fe3O4-C催化剂对甲醇、乙醇和丙醇氧化的电催化活性
收稿日期: 2016-11-04
修回日期: 2017-03-15
网络出版日期: 2017-03-16
基金资助
国家自然科学基金项目(21376070),湖南省研究生科研创新项目(CX2016B567)资助
Electroactivities of Pd/Fe3O4-C catalysts for electro-oxidation of methanol, ethanol and propanol
Received date: 2016-11-04
Revised date: 2017-03-15
Online published: 2017-03-16
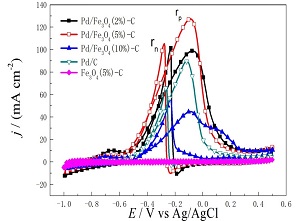
制备对醇氧化反应具有优异电活性的钯催化剂是醇燃料电池研究的重要内容。本文用硼氢化钠还原法制备了钯纳米颗粒, 然后沉积在Fe3O4/C复合物表面, 得到了不同Fe3O4负载量的Pd/Fe3O4-C催化剂. 透射电镜(TEM)图显示钯纳米颗粒均匀地分散在Fe3O4/C表面. 对制备好的Pd/Fe3O4-C催化剂进行了循环伏安法(CV)、计时电流(CA)和电化学阻抗谱(EIS)的测试, 研究了其在碱性介质中对C1-C3醇类(甲醇、乙醇和丙醇)氧化的电催化活性. 结果表明, 所制备的不同Fe3O4负载量的Pd/Fe3O4(2%)-C,Pd/Fe3O4(5%)-C, Pd/Fe3O4(10%)-C和Pd/C催化剂中, Pd/Fe3O4(5%)-C催化剂表现出最高的醇氧化电流密度. 依据循环伏安(CV)数据,Pd/Fe3O4(5%)-C催化剂对甲醇、乙醇、正丙醇和异丙醇氧化的阳极峰电流密度分别是Pd/C催化剂的1.7、1.4、1.7和1.3倍. Pd/Fe3O4(5%)-C催化剂对乙醇氧化的电荷传递电阻也远低于Pd/C催化剂. 制备的所有催化剂对C1-C3醇类电氧化的电流密度大小排序如下: 正丙醇﹥乙醇﹥甲醇﹥异丙醇. 此外, 碳粉中Fe3O4纳米颗粒的存在提高了钯纳米颗粒的电化学稳定性.
关键词: 钯催化剂; Fe3O4; 醇氧化; 电催化
邹涛,易清风,张媛媛,刘小平,徐国荣,聂会东,周秀林 . Pd/Fe3O4-C催化剂对甲醇、乙醇和丙醇氧化的电催化活性[J]. 电化学, 2017 , 23(6) : 708 -717 . DOI: 10.13208/j.electrochem.161104

Key words: Pd catalyst; Fe3O4; Alcohol oxidation; Electrocatalyst
/
| 〈 |
|
〉 |