

石英纳米通道单磷脂囊泡的电阻-脉冲分析方法
收稿日期: 2016-01-21
修回日期: 2017-03-13
网络出版日期: 2017-03-14
基金资助
We gratefully acknowledge financial supports from the National Institutes of Health (GM101133) and the University of Washington.
Resistive-Pulse Analysis of Single Phospholipid Vesicles Using Quartz Nanochannels
Received date: 2016-01-21
Revised date: 2017-03-13
Online published: 2017-03-14
Supported by
We gratefully acknowledge financial supports from the National Institutes of Health (GM101133) and the University of Washington.
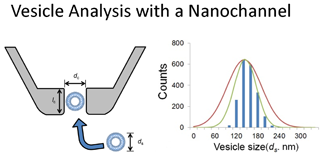
本文报告了用于检测单囊泡及其粒径分析的石英纳米通道电阻-脉冲分析方法. 采用圆柱形石英纳米通道可检测粒径为100~300 nm的单磷脂囊泡和直径为170~400 nm的聚苯乙烯纳米颗粒. 单囊泡和纳米颗粒的迁移可通过检测各自产生的方波电流脉冲信号, 并由此确定颗粒尺寸. 结果表明,采用石英纳米通道电阻-脉冲分析方法得到的颗粒/囊泡粒径与采用动态光散射法和扫描电子显微法得到的结果完全一致. 这种基于电子的分析方法具有快速简单的特点,所用的自制微传感器廉价耐用. 石英通道的应用还可与其它分析方法如电流分析法和荧光显微法联用,以获得生物囊泡及人工囊泡更完全的信息.
Jonathan T. Cox , 张波 . 石英纳米通道单磷脂囊泡的电阻-脉冲分析方法[J]. 电化学, 2017 , 23(2) : 207 -216 . DOI: 10.13208/j.electrochem.161250
We report the uses of resistive-pulse method and quartz nanochannels for the detection and size analysis of single vesicles. Cylindrical shape quartz nanochannels have been used to detect single phospholipid vesicles ranging from 100 to 300 nm and polystyrene nanoparticles ranging from 170 to 400 nm in diameter. Translocations of single vesicles and nanoparticle were detected as individual square current pulses, which could be used to determine particle size. Our results show excellent agreement between the particle/vesicle sizes obtained from nanochannels and those from dynamic light scattering (DLS) and scanning electron microscopy (SEM). This electronic-based method was found to be fast, simple, and used cheap and robust microsensors made in house. The application of a quartz channel might be combined with other analytical methods, such as amperometry and fluorescence microscopy, to yield more complete information about biological and artificial vesicles.

/
| 〈 |
|
〉 |