

添加剂丙烯基硫脲对镍电沉积的影响研究
收稿日期: 2016-12-20
修回日期: 2017-01-26
网络出版日期: 2017-02-13
基金资助
“973”国家重点基础研究发展计划项目(No. 2014CB643401)资助
Effect of allyl thiourea on nickel electrodeposition from solution containing ammonia and chloride
Received date: 2016-12-20
Revised date: 2017-01-26
Online published: 2017-02-13
采用循环伏安、线性扫描和恒电位阶跃电化学方法结合扫描电镜研究了不同浓度的丙烯基硫脲(ATU)对NH3-NH4Cl-H2O体系镍在玻碳电极上的电沉积过程的影响. 循环伏安测试、线性扫描以及恒电位暂态曲线一致表明ATU的加入对镍电沉积具有阻化作用,并且随着ATU浓度的增加其阻化作用增强;恒电位暂态曲线结果表明,镍的电结晶是按瞬时形核三维生长机理进行的,随外加电位负移,晶体向外生长速率增大;ATU的加入没有改变镍的形核方式,但形核数密度增大,并且减小晶体向外生长的速率;扫描电镜结果表明,ATU的加入可以细化晶粒,得到整平、致密的镍沉积层.
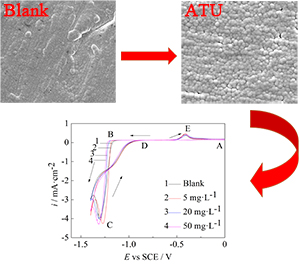
关键词: 镍电沉积; NH3-NH4Cl-H2O体系; 丙烯基硫脲; 添加剂
何亚宁 , 袁亮 , 丁治英 , 刘士军 . 添加剂丙烯基硫脲对镍电沉积的影响研究[J]. 电化学, 2017 , 23(6) : 638 -644 . DOI: 10.13208/j.electrochem.161217

Key words: nickel electrodeposition; NH3-NH4Cl-H2O solution; allyl thiourea; additive
/
| 〈 |
|
〉 |