

Nafion含量与阴离子吸附对于铂单原子层核壳结构催化剂制备的影响
收稿日期: 2016-12-26
修回日期: 2017-02-23
网络出版日期: 2017-02-23
基金资助
Financial supports to Lijun Yang’s post-doctoral fellowship by MITACS Accelerate Program and Ballard Power Systems are greatly appreciated.
Impact of Nafion Loading and Anion Adsorption on the Synthesis of Pt Monolayer Core-shell Catalysts
Received date: 2016-12-26
Revised date: 2017-02-23
Online published: 2017-02-23
Supported by
Financial supports to Lijun Yang’s post-doctoral fellowship by MITACS Accelerate Program and Ballard Power Systems are greatly appreciated.
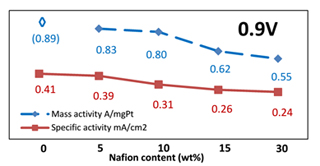
本实验利用铜的欠电位沉积技术,在旋转圆盘电极上以碳负载的钯纳米颗粒为核,制备铂单原子层核壳结构催化剂. 电化学测试用于表征不同Nafion含量的添加对于核壳结构催化剂制备的影响. 实验证明,Nafion的存在会影响铜的欠电位沉积,铂与铜的置换反应,并决定最终制备的核壳结构催化剂的氧还原催化反应的活性. 当催化剂薄层中Nafion的含量低于5%的时候,添加Nafion不但可以帮助催化剂附着在旋转圆盘电极表面,而且可以保证制备的催化剂具有较好的氧还原反应催化活性. 在H2SO4溶液中,钯纳米颗粒的表面存在特殊的阴离子吸/脱附电化学信号峰,这些信号峰可以用来监测Nafion含量对于铂单原子层核壳结构催化剂制备的影响.
杨莉君 , Dustin Banham , Elod Gyenge , 叶思宇 . Nafion含量与阴离子吸附对于铂单原子层核壳结构催化剂制备的影响[J]. 电化学, 2017 , 23(2) : 170 -179 . DOI: 10.13208/j.electrochem.161243
Carbon supported palladium (Pd) nanoparticles were used as a model core material for the synthesis of platinum (Pt) monolayer core-shell catalysts using rotating disk electrode method and a copper (Cu) under potential deposition technique. The impact of Nafion on the synthesis process was revealed by electrochemical testing with various Nafion contents. The existence of Nafion influenced the Cu under potential deposition, galvanic replacement and eventually the oxygen reduction reaction activity of the core-shell catalyst. However, as long as the Nafion content was less than 5 wt% in the test film, adding Nafion could help to bind catalyst onto the surface of electrode while maintaining promising catalytic activity. Unique anion adsorption/desorption peaks were observed on the surface of Pd in H2SO4 solution, which turned out to be a useful indicator to evaluate the impact of Nafion on the synthesis of the core-shell catalysts.

/
| 〈 |
|
〉 |