

Ce3+/Ce4+电对在混酸(硫酸和甲磺酸)中的电化学行为
收稿日期: 2016-08-27
修回日期: 2017-01-05
网络出版日期: 2017-01-10
基金资助
三门峡市科技攻关计划项目(No. 2016010108)资助
Electrochemical Behaviors of Ce3+/Ce4+ Couple in a Mixed-Acid Medium of CH3SO3H and H2SO4
Received date: 2016-08-27
Revised date: 2017-01-05
Online published: 2017-01-10
李照华 , 徐蛟龙 , 吴 婷 . Ce3+/Ce4+电对在混酸(硫酸和甲磺酸)中的电化学行为[J]. 电化学, 2017 , 23(6) : 718 -723 . DOI: 10.13208/j.electrochem.160827
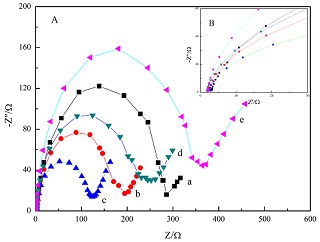
In order to determine the optimum molar ratio between methanesulfonic acid (MSA) and sulfuric acid, linear sweep voltammetry (LSV), cyclic voltammetry (CV), and electrochemical impedance spectroscopy (EIS) were used to investigate the electrochemical behaviors of cerium (III)/cerium (IV) (Ce3+/Ce4+couple on a Pt electrode in a mixed acid solution. Various concentrations of sulfuric acid were considered in the experiments, while the concentration of MSA was fixed at 1.0 mol·L-1. It was found that the optimum concentration of sulfuric acid was 0.8 mol·L-1 since the current response was the highest in the control groups and Ce3+ was easier to be oxidized to Ce4+. In the mixed acid solution, the solubility of cerium (III) increased to 0.95 mol·L-1 from 0.25 mol·L-1 in 1.3 mol·L-1 sulfuric acid solution. Electrolytic experiment and liquid-phase oxidation had been taken to explore the application of the mixed acid medium. The results revealed that Ce3+ exhibited the excellent electrochemical activity and Ce4+demonstrated a stronger oxidation capacity in the mixed acid solution.
Key words: mixed acid; current efficiency; solubility; current efficiency
/
| 〈 |
|
〉 |