

高烯丙醇和高烯丙胺的电化学合成研究进展
收稿日期: 2016-10-31
修回日期: 2017-01-16
网络出版日期: 2017-01-19
基金资助
国家自然科学基金(21471059)、华南理工大学探索性项目(Y1140410,Y9160040)资助
Electrochemical Synthesis of Homoallylic Alcohols and Homoallylic Amines
Received date: 2016-10-31
Revised date: 2017-01-16
Online published: 2017-01-19
钟伟强 , 梁向晖 , 黄精美 . 高烯丙醇和高烯丙胺的电化学合成研究进展[J]. 电化学, 2017 , 23(3) : 297 -306 . DOI: 10.13208/j.electrochem.161047
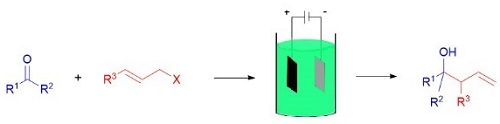
Electrochemical technique has been widely applied in the organic synthesis. This review focuses on the electrochemical synthesis of homoallylic alcohols and homoallylic amines from the allylation of carbonyl compounds and imines. This method has been developed impressively, especially in the field of electrochemical allylation in a green solvent of aqueous media. Improvement of the efficiency of the electricity, regio-selectivity and chiral synthesis are expected.
/
| 〈 |
|
〉 |