

单个MoS2纳米片氢析出催化特性研究
收稿日期: 2016-06-01
修回日期: 2016-07-20
网络出版日期: 2016-09-02
基金资助
国家自然科学基金项目(21173162)资助
Hydrogen Evolution Properties on Individual MoS2 Nanosheets
Received date: 2016-06-01
Revised date: 2016-07-20
Online published: 2016-09-02
高雨 , 周娟 , 刘欲文 , 陈胜利 . 单个MoS2纳米片氢析出催化特性研究[J]. 电化学, 2016 , 22(6) : 590 -595 . DOI: 10.13208/j.electrochem.160562
Molybdenum disulfide (MoS2) has been acknowledged to play important roles in hydrogen evolution reaction (HER) for hydrogen energy technology. Both computational and experimental results have suggested that the promising catalytic activity of MoS2 for the HER could be attributed to the sulfur edges of two-dimensional nanosheets, while their basal planes were catalytically inert. In order to verify this conclusion, we prepared single MoS2 sheet electrodes which were made of individual MoS2 sheets attached on the self-assembly monolayers (SAM) of SH(CH2)15COOH at Au ultramicroelectrodes (Au/SAM/MoS2). The single MoS2 sheet electrodes were prepared by dipping the SAM-modified Au ultramicroelectrodes in dilute solutions of MoS2 sheets whose sizes were similar to or slightly smaller than the Au/SAM electrodes. The electrocatalytic properties of the as-prepared single MoS2sheet electrodes with different sizes for HER were investigated in 0.5 mol·L-1 H2SO4. It is shown that the nanoscale MoS2 sheets exhibited superior HER activity over the microsize MoS2 sheets. This is because of the abundantly exposed active sites on the nanoscale MoS2 and the individual nanosheet could reflect its intrinsic reactivity more exactly. It directly proved that the active sites of MoS2 in HER were at the edges.
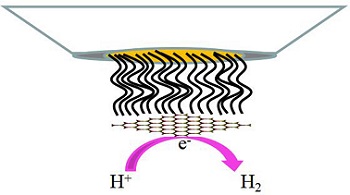
Key words: MoS2; edges; nanoelectrodes; hydrogen evolution reaction.
/
| 〈 |
|
〉 |