

锂硫电池用碳化钛涂层隔膜性能探究
收稿日期: 2016-06-06
修回日期: 2016-08-10
网络出版日期: 2016-09-28
基金资助
国家高技术研究发展计划(863计划)(No. 2015AA034600)资助
An Investigation in the Performance of Lithium Sulfur Battery with a TiC Coated Separator
Received date: 2016-06-06
Revised date: 2016-08-10
Online published: 2016-09-28
方建华 , 曹勇 , 杨茂萍 , 郑明森 , 董全峰 . 锂硫电池用碳化钛涂层隔膜性能探究[J]. 电化学, 2017 , 23(1) : 86 -90 . DOI: 10.13208/j.electrochem.160606
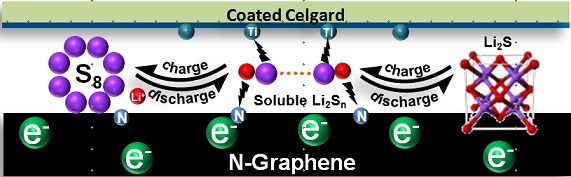
Key words: Li-S battery; TiC; Coating separator
/
| 〈 |
|
〉 |