

海水环境中典型阴离子对5083铝合金腐蚀行为的影响
收稿日期: 2016-06-14
修回日期: 2016-07-20
网络出版日期: 2016-08-02
基金资助
国家自然科学基金项目(51201169)资助
Influence of Typical Anions in Seawater Environments on Corrosion Behaviors of 5083 Aluminum Alloy
Received date: 2016-06-14
Revised date: 2016-07-20
Online published: 2016-08-02
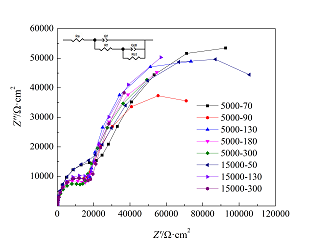
采用析因分析试验及动电位极化曲线、电化学阻抗谱(EIS)等测试方法,研究了海水环境因素中的典型阴离子(Cl-、 HCO3-、 SO42-交互作用对5083铝合金耐蚀性的影响. 结果表明,三种阴离子中,Cl-、HCO3-对铝合金点蚀起促进作用. Cl-与HCO3-交互作用时,在Cl-浓度一定的情况下,随着HCO3-浓度的增加,5083铝合金耐蚀性呈现出上升→下降→再上升的趋势,在70~90 mg•L-1时耐蚀性能明显降低;在HCO3-浓度一定的情况下,Cl-浓度较低时5083铝合金耐蚀性比Cl-浓度较高时差. 在Cl-、 HCO3-浓度较低情况下,SO42-具有抑制腐蚀的作用;当Cl-、 HCO3-浓度较高时,SO42-抑制腐蚀的作用不明显.
涂扬帆 , 冯立明 , 方志刚 , 刘海涛 , 井明华 , 管勇 . 海水环境中典型阴离子对5083铝合金腐蚀行为的影响[J]. 电化学, 2017 , 23(4) : 466 -472 . DOI: 10.13208/j.electrochem.160614

[1] Ezuber H, El-Houd A, El-Shawesh F. A study on the corrosion behavior of aluminum alloys in
seawater[J]. Materials and Design, 2008, 29: 801-805.
[2] Burstein G T, Liu C, Souto R M. Origins of pitting corrosion[J]. Corrosion Engineering, Science and Technology, 2004, 39(1): 25-30.
[3] Rowland H T, Dexter S C. Effect of sea water carbon dioxide system on the corrosion of aluminum[J]. Corrosion, 1980, 36(9): 458-467.
[4] Lin L Y (林乐耘), Zhao Y H (赵月红). Severe corrosivity and its eletrochemical mechanism of seawater in Xiamen sea area to Al-Mg alloys[J]. Journal of Electrochemistry (电化学), 2003, 9(3): 299-307.
[5] Mu Z J (穆振军),Lin Z J (林志坚),Zhuang Y (庄焱), et al. Corrosion behavior of aluminum-magnesium alloy in sea areas and its electrolyte effect in Xiamen sea area[J]. Development and application of materials (材料开发与应用), 2007, 22(5): 20-24.
[6] Huang G Q (黄桂桥). Corrosion of aluminium alloys in marine environment (Ⅰ)-a summary of 16 years exposure testing in seawater full immersion zone[J]. Corrosion & protection (腐蚀与防护), 2002, 23(1): 18-23
[7] Huang G Q (黄桂桥). Corrosion of aluminium alloys in marine environment (Ⅱ)-a summary of 16 years exposure testing in seawater full immersion zone[J]. Corrosion & protection (腐蚀与防护), 2002, 23(2): 47-50.
[8] Huang G Q (黄桂桥). Corrosion of aluminium alloys in marine environment (Ⅲ)-a summary of 16 years exposure testing in seawater full immersion zone[J]. Corrosion & protection (腐蚀与防护), 2003, 24(2): 47-57.
[9] Box G E P, Hunter J S, Hunter W G. Statistics for experimenters: Design, Innovation, and Discovery, Second Edition[M]. (Zhang R C (张润楚), Liu M Q (刘民千), Yang J F (杨建峰), et al, Trans.). Beijing: China Machine Press, 2009. 105.
[10] Chen K (陈魁). Design and analysis of experiments[M]. Beijing: Tsinghua University Press, 2005. 61.
[11] Kim S J, Jang S K, Kim J I. Investigation on optimum corrosion protection potential of Al alloy in marine environment[J]. Materials Science-Poland, 2008, 26(3): 779-785.
[12] Juttner K. Electrochemical Impedance Spectroscopy (EIS) of corrosion processes on inhomogeneous surface[J], Electrochemical Acta, 1990, 35: 1501.
[13] Peng W C (彭文才), Hou J (侯健), Guo W M (郭为民), et al. Effect of temperature and dissolved oxygen on corrosion performance of alloy 5083 in seawater[J]. Equipment Environment Engineering (装备环境工程), 2010, 7(3): 22-26.
[14] Moreto J A, Marino C E B, Filho W W B. SVET, SKP, and EIS study of the corrosion behaviour of high strength Al and Al-Li alloys used in aircraft fabrication[J]. Corrosion Science, 2014, 84: 31-41.
/
| 〈 |
|
〉 |