

晶态Li12Si7锂离子电池负极材料的电化学性能研究
收稿日期: 2016-05-03
修回日期: 2016-05-19
网络出版日期: 2016-07-28
基金资助
国家自然科学基金资助项目(51471152),教育部创新团队项目(IRT13037)和中组部拔尖人才支持
Electrochemical Performance of Crystalline Li12Si7 as Anode Material for Lithium Ion Battery
Received date: 2016-05-03
Revised date: 2016-05-19
Online published: 2016-07-28
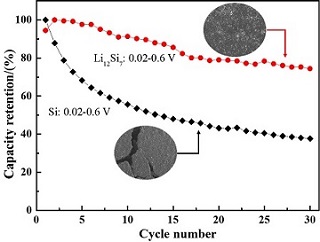
通过加热摩尔比为12:7的LiH/Si球磨混合物,避免了Li与Si之间巨大的熔点差异,成功制备了晶态Li12Si7合金,研究了其电化学性能和储锂机制. 发现Li12Si7在0.02 ~ 0.6 V的嵌脱锂过程中,只发生晶胞体积的变化,而不产生相变,呈现出明显的固溶储锂机制. 该固溶储锂机制的存在,有效抑制了Si基负极材料嵌脱锂过程中由于相变导致的体积效应,使得晶态Li12Si7在0.02 ~ 0.6 V电压范围内具有显著改善的电化学性能,其首次库伦效率高达100%,30次循环后的可逆容量保持率约为74%,分别优于相同条件下原始Si电极的55%和37%.
杨亚雄 , 马瑞军 , 高明霞 , 潘洪革 , 刘永锋 . 晶态Li12Si7锂离子电池负极材料的电化学性能研究[J]. 电化学, 2016 , 22(5) : 521 -527 . DOI: 10.13208/j.electrochem.160541
Crystalline Li12Si7 is successfully synthesized by heating the mixture of LiH and Si with a molar ratio of 12:7, which avoids the huge difference of the melting points between Li and Si. The electrochemical performance and lithium storage mechanism of the as-prepared Li12Si7 are studied in this work. It is found that only a change in cell volume takes place without a phase change during the lithiation/delithiation of Li12Si7 at a voltage range of 0.02 ~ 0.6 V, exhibiting a solid-solution lithium storage mechanism. Such a lithium storage process effectively retards the volume effect caused by the phase change during lithiation/delithiation of Si-based anode. This induces significantly the improved electrochemical properties of crystalline Li12Si7 while cycling at 0.02 ~ 0.6 V. The first Coulombic efficiency of crystalline Li12Si7 is determined to be as high as 100%, and the capacity retention is 74% after 30 cycles, which are distinctly higher than those of Si anode (55% and 37%, respectively) under identical conditions.

/
| 〈 |
|
〉 |