

阴极界面修饰层改善平面p-i-n型钙钛矿太阳能电池的光伏性能
收稿日期: 2016-06-30
修回日期: 2016-07-21
网络出版日期: 2016-07-22
基金资助
国家自然科学基金项目(No. 91333204, No. 91433117)资助
Cathode Buffer Layer for Improving Photovoltaic Performance of Planar p-i-n Perovskite Solar Cells
Received date: 2016-06-30
Revised date: 2016-07-21
Online published: 2016-07-22
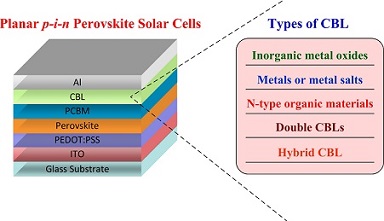
有机/无机杂化金属卤化物钙钛矿半导体材料结合了有机材料良好的溶液可加工性以及无机材料优越的光电特性,近几年受到了热捧,成为太阳能电池领域一颗耀眼的明星. 伴随着钙钛矿薄膜结晶过程和形貌的优化、器件结构的改进以及电极界面材料的开发,这类有机/无机杂化金属卤化物钙钛矿太阳能电池的光电转换效率从最初的3.8%迅速提高到目前最高的22.1%. 其中界面工程在提升器件性能上发挥着极其重要的作用. 本文总结了平面p-i-n型钙钛矿太阳能电池中阴极界面修饰层(CBL)的研究进展. CBL从材料上讲可分为无机金属氧化物、金属或金属盐以及有机材料,从构成上讲可分为单层CBL、双层CBLs以及共混型CBL. 本文对这些类型的CBL分别给予详细的介绍. 最后,我们归纳出CBL在改善器件效率和稳定性上所起的作用以及理想CBL所应满足的要求,希望能为以后阴极界面修饰材料的设计提供一定的借鉴.
刘晓东 , 李永舫 . 阴极界面修饰层改善平面p-i-n型钙钛矿太阳能电池的光伏性能[J]. 电化学, 2016 , 22(4) : 315 -331 . DOI: 10.13208/j.electrochem.160148
Organic/inorganic hybrid metal halide perovskite semiconductor materials have drawn great attention for the application in solar cells in recent years because of their combined superior photoelectrical properties of inorganic semiconductors (with high dielectric constant and high charge carriers mobility) and organic semiconductors (with good solution processability and high absorbance). The power conversion efficiency (PCE) of the organometal halide perovskite solar cells (pero-SCs) based on CH3NH3PbI3 has been increased dramatically in a few years from 3.8% to a certified 22.1%, primarily owing to the development of new interfacial materials, careful optimization of morphology, and perovskite crystallization processes of the active layers and the device architecture. Among the optimization strategies, interface engineering plays a vital role in improving photovoltaic performance of the pero-SCs. Organometal halide perovskite material CH3NH3PbI3 was first used in solar cells in 2009 as a sensitizer in dye-sensitized solar cells with a PCE of 3.81%, and then the PCE was improved to 6.54% in 2011. However, the stability of the solar cells with a liquid electrolyte is very poor due to the easy decomposition of the perovskite in the liquid electrolyte. In 2012, spiro-MeOTAD was used as a solid hole transporting layer on the perovskite layer instead of liquid electrolyte, and all solid state pero-SCs were fabricated. The solid state pero-SCs based on mesoporous TiO2 electrode showed higher PCE of 9.7% with much improved stability. Later, the planar structured pero-SCs were developed with the dense planar electrode as a cathode. Now the planar structured pero-SCs can be classified into planar n-i-p pero-SCs with a cathode buffer layer (CBL) on a transparent electrode and p-i-n pero-SCs with an anode buffer layer on a transparent electrode. In this review article, we summarized the latest development of CBLs for highly efficient and stable planar p-i-n pero-SCs. The CBL materials can be divided into inorganic metal oxides, metals or metal salts, and n-type organic semiconductor materials according to the types of materials. And the types of the CBLs can be classified into single CBL, double CBLs, and hybrid CBL according to the CBL composition. The effects of the CBLs on the photovoltaic performance and device stability of the pero-SCs were reviewed systematically. Finally, we summarized the effects of CBL on the improvements of device efficiency and stability as well as the requirements for an ideal CBL. We hope that the properties and requirements of the ideal CBLs we summarized in this article will provide guidance for the future molecular design of cathode interfacial materials.

/
| 〈 |
|
〉 |