

Sn掺杂对氧空位型α-Fe2O3纳米颗粒光解水性能的影响
收稿日期: 2016-04-12
修回日期: 2016-05-06
网络出版日期: 2016-05-18
基金资助
国家自然科学基金项目(21303053)和化学工程联合国家重点实验室开放基金(SKLChE-14C02)资助
Sn-Doped α-Fe2O3 Photocatalyst containing Oxygen Vacancy for Water-splitting
Received date: 2016-04-12
Revised date: 2016-05-06
Online published: 2016-05-18
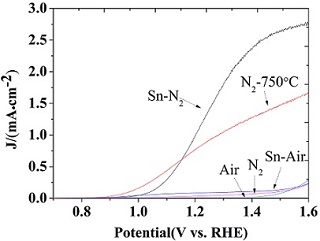
在退火前未抽真空条件下,采用滴涂法在常压氮气氛围中退火制备了含氧空位的α-Fe2O3纳米颗粒. 通过在空气和氮气氛围中退火和向前驱体溶液直接加入SnCl4制备α-Fe2O3的方法研究了Sn掺杂对氧空位型α-Fe2O3纳米颗粒光催化性能的影响. 结果表明,氮气氛围中退火Sn掺杂得到的α-Fe2O3在1.23V vs. RHE时的电流密度分别是氮气氛围中退火未掺杂α-Fe2O3的35倍和空气氛围中退火Sn掺杂α-Fe2O3的15倍,氮气氛围中退火和掺杂被证明是获得高催化性能必不可少的条件. Mott-Schottky曲线和交流阻抗谱表明,掺杂和氧空位能增大催化剂的载流子浓度的电导率. 在牺牲剂溶液中测试发现,Sn掺杂导致材料的表面反应速率提高是催化剂活性的重要影响因素.
关键词: α -Fe2O3;Sn掺杂;氧空位;表面反应速率
王祖华 , 钮东方 , 李辉成 , 杜荣斌 , 徐衡 , 张新胜 . Sn掺杂对氧空位型α-Fe2O3纳米颗粒光解水性能的影响[J]. 电化学, 2017 , 23(1) : 21 -27 . DOI: 10.13208/j.electrochem.160412
The α-Fe2O3 nanoparticles containing oxygen vacancies were synthesized in atmospheric N2 by dip-dropping method without a high vacuum employed before annealing. The influences of annealing atmosphere and Sn-doping on the photocatalytic performance of α-Fe2O3 nanoparticles were studied by annealing the photocatalyst in N2 or air and adding SnCl4 to the precursor directly. The results showed that the current density of Sn-doping α-Fe2O3 annealed in N2 at 550 °C and 1.23 V (vs. RHE) was 35 times greater than that of pristine α-Fe2O3 annealed in N2 at 550 °C and 15 times greater than that of Sn-doping α-Fe2O3 annealed in air at 550 °C, which indicated that both Sn-doping and annealing in N2 were indispensible to obtain a good performance for α-Fe2O3 nanoparticles. Mott-Schottky curves and electrochemical impedance spectroscopic data proved that both Sn-doping and oxygen vacancy could lead to the increase of the donors concentration and conductivity, which resulted in the enhanced performance of α-Fe2O3 nanoparticles. The photocatalytic performance tested in the electrolyte containing sacrifice solvent confirmed that the Sn-doping could facilitate the surface reaction, which was another key factor contributed to the enhanced performance of α-Fe2O3 nanoparticles.

Key words: α-Fe2O3 photocatalyst; oxygen vacancy; Sn doping; surface reaction rate
[3] Yang J M(杨加明), Han L J(韩玲军), Zhong L P(钟丽萍), et al. Preparation and photocatalytic Properties of ZnO Nanorod Arrays on Ti substrates [J]. Journal of electrochemistry(电化学), 2014, 03): 288-292
[4] Wheeler D A, Wang G, Ling Y, et al. Nanostructured hematite: Synthesis, characterization, charge carrier dynamics, and photoelectrochemical properties[J]. Energy & Environmental Science, 2012, 5(5): 6682-6702
[13] Ling Y, Wang G, Reddy J, et al. The influence of oxygen content on the thermal activation of hematite nanowires[J]. Angewandte Chemie International Edition, 2012, 51(17): 4074-4079
[14] Morrish R, Rahman M, MacElroy J M D, et al. Activation of hematite nanorod arrays for photoelectrochemical water splitting[J]. ChemSusChem, 2011, 4(4): 474-479
/
| 〈 |
|
〉 |