

光电化学分解水电池的电极性能提高方法及光阴极研究进展
收稿日期: 2016-03-18
修回日期: 2016-04-28
网络出版日期: 2016-05-05
基金资助
国家重点基础研究发展计划(973项目,2015CB932200与2014CB239303)、江苏省高校自然科学研究面上项目(15KJB150010) 、有机电子与信息显示国家重点实验室培育基地开放课题、南京工业大学校内课题的资助.
Photoelectrochemical Water Splitting cells: Methods for Improving Performance of Electrodes and Recent Progress on Photocathodes
Received date: 2016-03-18
Revised date: 2016-04-28
Online published: 2016-05-05
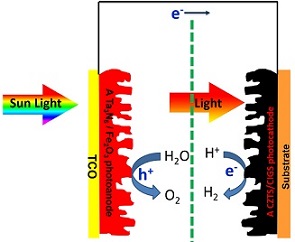
光电化学水分解电池能够将太阳能直接转化为氢能,是一种理想的太阳能利用方式. p-n叠层电池具有理论转换效率高、成本低廉、材料选择灵活等优势,被认为是最有潜力的一类光电化学水分解电池. 然而,目前这类叠层电池的太阳能转化效率还不高,主要原因是单个电极的效率太低. 本文介绍了几种提高光电极分解水性能的方法——减小光生载流子的体相复合、表面复合以及抑制背反应等,同时综述了国内外关于几种p型半导体光阴极的研究进展,如Si、InP、CuIn1-x GaxS(Se)2、Cu2ZnSnS4等. 通过总结,作者提出一种p-Cu2ZnSnS4(CuIn1-xGaxS(Se)2)/n-Ta3N5(Fe2O3) 组装方式,有望获得高效低成本叠层光电化学水分解电池.
朱凯健 , 罗文俊 , 关中杰 , 温鑫 , 邹志刚 , 黄维 . 光电化学分解水电池的电极性能提高方法及光阴极研究进展[J]. 电化学, 2016 , 22(4) : 368 -381 . DOI: 10.13208/j.electrochem.160147
Photoelectrochemical water splitting can convert solar energy into hydrogen which is an ideal way to utilize and store solar energy. A p-n tandem cell is considered as the most promising solar water splitting cell due to its high theory conversion efficiency, low cost and photoelectrode material flexibility. However, solar conversion efficiency of a tandem cell is still low in the experiment because of poor performance in a single photoelectrode. In this review, we have introduced some effective approaches to improve the performances of photoelectrodes by reducing recombination of photogenerated carriers in the bulk or on the surface, and suppressing back reaction. Moreover, we have also summarized recent progress of some p-type semiconductor photocathodes, such as Si, InP, CuIn1-x GaxS(Se)2 and Cu2ZnSnS4. Accordingly, we constructed a promising p-Cu2ZnSnS4(CuIn1-xGaxS(Se)2)/n-Ta3N5(Fe2O3)photoelectrode and obtained an efficient photoelectrochemical tandem cell with low cost.

/
| 〈 |
|
〉 |