

超薄Co3O4纳米片薄膜制备及其电化学传感器性能
收稿日期: 2016-01-24
修回日期: 2016-04-12
网络出版日期: 2016-05-20
基金资助
河北省自然科学基金项目(B2015203350)和秦皇岛市科技支撑计划项目(201602A004)资助
Synthesis of Ultrathin Co3O4 Nanoflakes Film Material for Electrochemical Sensing
Received date: 2016-01-24
Revised date: 2016-04-12
Online published: 2016-05-20
王慧娟 . 超薄Co3O4纳米片薄膜制备及其电化学传感器性能[J]. 电化学, 2016 , 22(6) : 631 -635 . DOI: 10.13208/j.electrochem.160124
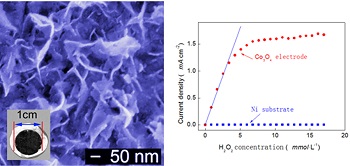
Ultrathin cobalt oxide (Co3O4 ) nanoflakes film material was synthesized by using an electro-deposited cobalt layer as a raw material through a simple oxidation method and followed by a heat treatment at 350 oC. The physical characterizations of the Co3O4 nanoflakes film were performed by scanning electron microscopy (SEM), X-ray diffraction (XRD) and transmission electron microscopy (TEM) technologies, and the electrochemical activity was measured by cyclic voltammetry (CV). As a promising material for electrochemical sensing, the as-synthesized ultrathin Co3O4 nanoflakes film material exhibited excellent electrochemical activity for H2O2 with a wide linear detection range (0 ~ 4 mmol•L-1) and high sensitive current response (~ 1.15 mA•cm-2).
/
| 〈 |
|
〉 |