

H型电解池中CO2电化学还原的阳极电解液问题
收稿日期: 2016-01-21
修回日期: 2016-06-14
网络出版日期: 2016-06-20
基金资助
江苏省生态建材与环保装备协同创新中心和江苏省新型环保重点实验室联合资助(No. GX2015105)
The Problem of the Anode Electrolyte in H-Type Electrolytic Cell for Electrochemical Reduction of Carbon Dioxide
Received date: 2016-01-21
Revised date: 2016-06-14
Online published: 2016-06-20
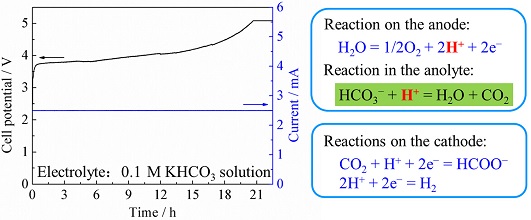
采用自制的H型电解池开展了KHCO3溶液中电化学还原CO2制甲酸的研究. 研究发现,在电解池中长时间电解时阴阳两极间的电压(槽电压)会持续升高,导致电解过程不可持续. 经过恒电位电解、恒电流电解、pH测试以及电解前后阳极室KHCO3浓度分析等实验研究,作者发现,这是由以下过程引起的:阳极上的析氧反应产生的H+与电解液中的HCO3-反应生成水和CO2,导致阳极室的HCO3-的消耗,之后阳极室的K+被迫扩散进入阴极室而导致阳极室电解质浓度下降. 因此,阳极室电解液导电性下降,进而引起阳极电位的升高. 研究发现,阳极电解液具有碱性时,都可能发生此种现象,因此,为了保证电解过程可持续且保持高的能量转换效率,阳极液的电解质不能是任何具有碱性的物质.
张 瑞 , 吕伟欣 , 雷立旭 . H型电解池中CO2电化学还原的阳极电解液问题[J]. 电化学, 2017 , 23(1) : 72 -79 . DOI: 10.13208/j.electrochem.160121
Electrochemical reduction of carbon dioxide (CO2) was studied in the H-type electrolytic cell. It was found that the voltage between the cathode and the anode would increase during the long time electrolysis process, for this reason the electrolytic process would be unsustainable. After the experimental investigations carried out by constant potential electrolysis, constant current electrolysis, pH test and KHCO3 concentration analysis of anode electrolyte before and after the electrolysis, the increase in cell voltage might be caused by the following process: H+, that was generated from the anodic oxygen evolution reaction, reacted with HCO3- to form water and CO2, and the HCO3-in the anode chamber was consumed; then K+ in the anode chamber was forced to spread into the cathode chamber which led to the decrease of the electrolyte concentration in the anode chamber. Therefore, the conductivity of the electrolyte solution in the anode chamber decreased, resulting in the rise of the anode potential. This phenomenon may happen in the alkaline electrolyte in an anode cell, therefore, in order to ensure the electrolysis process sustainable and keep high energy conversion efficiency, the anode electrolyte cannot be any alkaline substance.

/
| 〈 |
|
〉 |