

富锂锰基材料xLi2MnO3•(1-x)LiMn1/3Ni1/3Co1/3O2(x = 0.3,0.5,0.7)的电化学和同步辐射研究
收稿日期: 2016-03-16
修回日期: 2016-05-25
网络出版日期: 2016-06-02
基金资助
国家自然科学基金项目(No. 2011CB935903)和上海市科委项目(No. 13JC1407900)资助
Electrochemical and in situ X-ray Absorption Fine Structure Study of Li-Rich Cathode Materials
Received date: 2016-03-16
Revised date: 2016-05-25
Online published: 2016-06-02
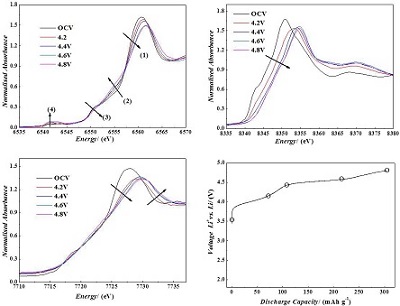
采用共沉淀的方法,以过渡金属硫酸盐为起始物质制备了一系列不同组成的富锂锰基正极材料xLi2MnO3•(1-x)LiMn1/3Ni1/3Co1/3O2(x = 0.3,0.5,0.7),通过XRD、Rietveld精修等物理手段比较了不同组成材料的结构特征. 通过对比不同比例材料的首周库仑效率、放电可逆容量、循环性能、电压降现象及不同温度下各比例富锂材料的倍率表现等电化学性能,确定 0.5Li2MnO3•0.5LiMn1/3Ni1/3Co1/3O2为该系列材料的最优比例. 然后采用原位X射线吸收谱技术,对富锂材料在首周活化过程中的机理进行了研究. 同步辐射结果表明,在首周充电过程中,镍和钴的价态分别从+2、+3价氧化到+4价,而对于锰来讲,虽然在富锂锰基材料活化的过程中其周围的局域电子结构发生了一定的变化,但是其化合价始终维持在+4价没有发生变化.
侯孟炎 , 鲍洪亮 , 王 珂 , 王建强 , 夏永姚 . 富锂锰基材料xLi2MnO3•(1-x)LiMn1/3Ni1/3Co1/3O2(x = 0.3,0.5,0.7)的电化学和同步辐射研究[J]. 电化学, 2016 , 22(3) : 288 -298 . DOI: 10.13208/j.electrochem.151248
A series of the lithium-rich and manganese-based layered structure xLi2MnO3•(1-x)LiMn1/3Ni1/3Co1/3O2 (x = 0.3,0.5,0.7) materials were synthesized by a co-precipitation method, and followed by a solid-state reaction process. By comparing the first cycle efficiency, the reversible discharge capacity, the cycling stability and the voltage decay during the charge/discharge cycling process, the material with the composition of 0.5Li2MnO3•0.5LiMn1/3Ni1/3Co1/3O2was found to show the best electrochemical performance. The lithium storage mechanism and thermal stability of the de-lithiated compound were also investigated by in situ X-ray absorption fine structure (XAFS) spectroscopy and differential scanning calorimetry (DSC) techniques. The results of XAFS indicates that during the charging process to 4.5 V, the Ni and Co ions are oxidized to Ni4+ and Co4+, respectively, while the Mn ion remains Mn4+.

/
| 〈 |
|
〉 |