

Pt/C催化剂氧还原反应的交流阻抗动态研究
收稿日期: 2016-03-10
修回日期: 2016-04-10
网络出版日期: 2016-04-27
基金资助
国家自然科学基金项目(51273103, 50873050, 51573084)、国家973计划(2012CB215500)资助
The Study of Dynamical Electrochemical Impedance Spectroscopy for Oxygen Reduction Reaction on Pt/C Catalyst
Received date: 2016-03-10
Revised date: 2016-04-10
Online published: 2016-04-27
史坤明 , 郭建伟 , 王佳 . Pt/C催化剂氧还原反应的交流阻抗动态研究[J]. 电化学, 2016 , 22(5) : 542 -548 . DOI: 10.13208/j.electrochem.160310
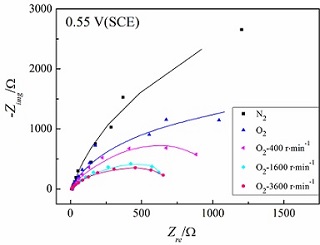
With joint techniques of rotating disc electrode(RDE) and electrochemical impedance spectroscopy(EIS), and further establishment on equivalent circuit model, this paper studied oxygen reduction reaction(ORR) on commercial Pt/C catalyst in acid medium. Our results found that the dynamical interface on Pt/C consists of two independent processes: 1) the PtO reduction from Pt surface, 2) the new PtO formation from ORR, thus providing key clues for catalyst stability and activity. This also implied that the dynamical interface facilitates reconstruction for porous electrode, and matches with mass transfer. One important issue is discovered that at high overpotential, the high reaction rate for ORR can be further improved if providing hydrophobicity on catalyst surface. All these efforts on ORR progress not only compensate for DC electrochemistry study, but also provide basis for future model establishment.
/
| 〈 |
|
〉 |