

担载型纳米金的制备参数优化及电催化性能探索
收稿日期: 2016-03-05
修回日期: 2016-03-12
网络出版日期: 2016-04-28
基金资助
国家自然科学基金项目(No. 21003114,No. 21373211,No. 21103163,No. 21306188,No. 21306187)、2015辽宁省“百千万人才工程”(辽百千万立项[2015]19号)、2015“辽宁省高等学校优秀科技人才支持计划”(No. LR2015014)、2015“大连市杰出青年科技人才”(No. 2015R006)及“中央高校基本科研业务费专项资金”(No. DUT15ZD225,No. DUT15RC(3)001)资助
Preparation Parameters Optimization and Electrocatalytic Properties of Supported Au Nanoparticles
Received date: 2016-03-05
Revised date: 2016-03-12
Online published: 2016-04-28
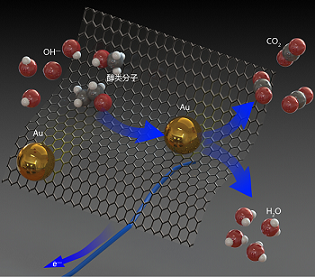
纳米尺度的金由于常表现出有趣的尺寸效应和物理化学特性而被大量应用于催化反应中,但是其在电催化反应中的应用却十分有限. 本文以水为溶剂、HAuCl4为前驱体、十二烷基聚乙二醇醚(Brij 35)等为软模板剂、NaBH4为还原剂、活性炭或石墨烯为载体,在温和反应条件下获得担载型金纳米电催化剂. 本文考察并优化了关键制备参数和样品纯化方法,最终确定NaBH4的最佳浓度区间为5 ~ 10 mmol•L-1,Brij 35的最佳浓度约为1 mmol•L-1,在3 ~ 16 oC下金纳米颗粒的尺寸容易控制,石墨烯和活性炭(EC600)是金纳米颗粒的良好载体. 在优化的反应条件下,金纳米颗粒的粒径可以被控制在1 ~ 4 nm. 热处理法可以有效去除表面活性剂,纯化后的担载型纳米金电催化剂在醇类小分子的氧化反应中表现出良好的性能.
姚 瑞 , 宋玉江 , 李焕巧 , 李 佳 , 刘建国 . 担载型纳米金的制备参数优化及电催化性能探索[J]. 电化学, 2016 , 22(2) : 147 -156 . DOI: 10.13208/j.electrochem.151151
Due to interesting size effect, physical and chemical properties, nano-scale gold materials have been commonly used to catalytic reactions. However, the application of gold nanomaterials in the field of electrocatalysis is limited. Herein, we report the synthesis of gold nanparticles supported on carbon through chemical reduction of HAuCl4 by NaBH4 under mild conditions in the presence of surfactants as soft templates, carbon black or graphene as a support. We investigated a series of key reaction parameters, including reagent concentration, temperature, the types of carbon supports and surfactants. With the optimum synthetic parameters, we successfully obtained supported 1 ~ 4 nm gold particles. In addition, we found that the heat treatment could effectively remove such a surfactant as Brij35. The purified electrocatalysts demonstrated electrocatalytic activities toward both oxygen reduction reaction and alcohol oxidation reaction.

Key words: synthesis; supported gold nanoparticles; electrocatalysis
[2] Haruta M. Catalysis - gold rush[J]. Nature, 2005, 437(7062): 1098-1099.
[6] Gong J L, Flaherty D W, Yan T, et al. Selective oxidation of propanol on Au(111): Mechanistic insights into aerobic oxidation of alcohols[J]. ChemPhysChem, 2008, 9(17): 2461-2466.
[12] Xie Y, Li H Q, Tang C Z, et al. A high-performance electrocatalyst for oxygen reduction based on reduced graphene oxide modified with oxide nanoparticles, nitrogen dopants, and possible metal-N-C sites[J]. Journal of Materials Chemistry A, 2014, 2(6): 1631-1635.
[13] Si W F, Li J, Li H Q, et al. Light-controlled synthesis of uniform platinum nanodendrites with markedly enhanced electrocatalytic activity[J]. Nano Research, 2013, 6(10): 720-725.
[14] Li S S, Li H Q, Zhang Y S, et al. One-step synthesis of carbon-supported foam-like platinum with enhanced activity and durability[J]. Journal of Materials Chemistry A, 2015, 3(43): 21562-21568.
[15] Xie Y, Tang C Z, Wang A J, et al. Self-assembled nanoporous hemin with high density of ordered active sites and large surface area for oxygen reduction reaction[J]. Faraday Discuss, 2014, 176: 393-408.
[16] Li H Q, Yao R, Wang D, et al. Facile synthesis of carbon supported Pd3Au@super-thin Pt core/shell electrocatalyst with a remarkable activity for oxygen reduction[J]. Journal of Physical Chemistry C, 2015, 119(8): 4052-4061.
[17] Li S S, Liu H Y, Wang Y, et al. Controlled synthesis of high metal loading electrocatalysts with significantly enhanced activity and durability toward oxygen reduction reaction[J]. RSC Advances, 2015, 5(12): 8787-8792.
[18] Li L(李莉), Wei Z D(魏子栋). Electrochemical catalysis: A DFT study[J]. Journal of Electrochemistry(电化学), 2014, 20(4): 307-315.
[19] Nguyen T G H, Pham T V A, Phuong T X, et al. Nano-Pt/C electrocatalysts: Synthesis and activity for alcohol oxidation[J]. Advances in Natural Sciences: Nanoscience and Nanotechnology, 2013, 4: 035008.
/
| 〈 |
|
〉 |