

钼基析氢反应电催化剂的研究进展
收稿日期: 2015-12-10
修回日期: 2016-01-27
网络出版日期: 2016-04-28
基金资助
国家自然科学基金项目(No. 21306060,No. 21573083)、教育部新世纪优秀人才支持计划(No. NCET 130237)资助
Recent Progresses in Molybdenum-Based Electrocatalysts for the Hydrogen Evolution Reaction
Received date: 2015-12-10
Revised date: 2016-01-27
Online published: 2016-04-28
吴则星 , 王 杰 , 郭军坡 , 朱 静 , 王得丽 . 钼基析氢反应电催化剂的研究进展[J]. 电化学, 2016 , 22(2) : 192 -204 . DOI: 10.13208/j.electrochem.151143
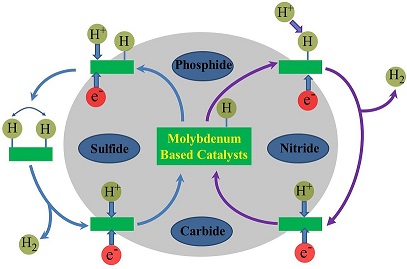
Electrochemical catalytic production of hydrogen has been considered as a promising and sustainable strategy for clean and renewable energy technologies. Molybdenum-based non noble metal catalysts for the hydrogen evolution reaction have attracted extensive attention due to its effective catalytic performance. In this review, the recent progresses in molybdenum-carbide, phosphide, nitride and sulfide electrocatalysts are presented. In addition, the strategies to improve the catalytic performance are analyzed and the prospects for the future development trends are expected.
/
| 〈 |
|
〉 |