

质子交换膜燃料电池Pt/C阴极氧还原动力学模拟
收稿日期: 2016-01-05
修回日期: 2016-02-19
网络出版日期: 2016-04-28
基金资助
国家自然科学基金项目(21476178),博士点基金优先发展领域项目(20130143130001)资助
Dynamic Simulation of Oxygen Reduction Reaction in Pt/C Electrode for Proton Exchange Membrane Fuel Cells
Received date: 2016-01-05
Revised date: 2016-02-19
Online published: 2016-04-28
李 赏 , 周 芬 , 陈 磊 , 潘 牧 . 质子交换膜燃料电池Pt/C阴极氧还原动力学模拟[J]. 电化学, 2016 , 22(2) : 129 -134 . DOI: 10.13208/j.electrochem.151150
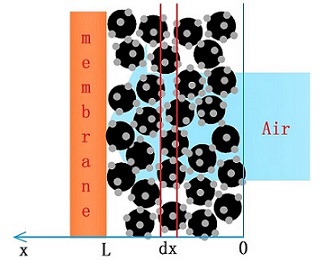
It is an urgent need to reduce the Pt loading in electrode for practical fuel cell applications worldwide. Herein, we theoretically investigate the oxygen distribution, generated current, and minimum Pt loading of Pt/C electrode for practical applications based on kinetic model of oxygen reduction reaction. The results indicate that with increasing electrode effective thickness to 40 mm, serious concentration polarization is expected for Pt/C electrode. To generate a power density of 1.4 W•cm-2 (2.1A•cm-2 @0.67 V) for fuel cell, the cathode catalyst layer thickness in PEMFC should be as thin as 3 mm. The minimum Pt loading will reach 0.122 mg•cm-2, which can reduce the amount of Pt to 0.087 g•kW-1 in PEMFC cathode.
Key words: Pt/C electrode; oxygen reduction; mass transport; Pt loading; fuel cell
/
| 〈 |
|
〉 |