

纳米Fe(OH)3为模板的三维石墨烯类多孔碳的制备及其催化氧还原性能研究
收稿日期: 2015-12-10
修回日期: 2016-01-18
网络出版日期: 2016-04-28
基金资助
国家自然科学基金(Nos. 21276148 和 21076119) 和化学工程国家重点实验室(天津大学)开放课题(No. SKL-ChE-14B01)资助
Preparation and Performance of 3D Graphene Type Porous Carbon Employing Nano Fe(OH)3 as Template for Oxygen Reduction Catalyst
Received date: 2015-12-10
Revised date: 2016-01-18
Online published: 2016-04-28
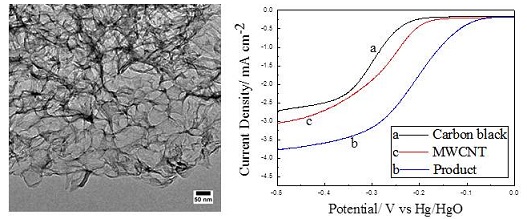
以煤焦油沥青为碳源,纳米Fe(OH)3为模板制备了一种三维石墨烯类多孔碳材料,通过测试氧还原性能,确定了最佳制备工艺为:反应物煤沥青,纳米Fe(OH)3,KOH的质量配比为6:8:4,热解温度为800 ℃. 扫描电镜(SEM)测试结果表明,制得的产品具有明显的孔结构且分布均匀. 透射电镜(TEM)测试结果进一步表明,产品具有泡沫状的多孔结构,高分辨透射电子显微镜图像表明该产品具有多层的三维石墨烯结构. X射线衍射(XRD)数据表明,在29o位置出现的衍射峰是多层石墨烯结构,42o位置的衍射峰表明,产品具有一定程度的石墨化. 由拉曼光谱结果计算IG与I2D的比值表明产品为多层石墨烯结构. X射线光电子能谱分析(XPS)检测到的C元素含量约为88.7%,主要包含C-C键,图谱中未发现铁元素的存在,证明纳米Fe(OH)3模板已被洗净. 根据比表面积测定(BET)可知,多孔碳的比表面积为2040 m2•g-1,孔径集中分布在10~400 nm,这与TEM测试得到的结果一致. 在0.1 mol•L-1 KOH中进行催化氧还原性能测试,起始还原电位为0 V (vs. Hg/HgO),电子转移数为3.58。测试结果表明,制得的三维石墨烯类多孔碳具有良好的催化氧还原性能.
孙崇云 , 李忠芳 , 卢雪伟 , 钟西站 , 刘玉荣 . 纳米Fe(OH)3为模板的三维石墨烯类多孔碳的制备及其催化氧还原性能研究[J]. 电化学, 2016 , 22(2) : 157 -163 . DOI: 10.13208/j.electrochem.151142
The 3D graphene type porous carbon was prepared using coal tar pitch as carbon source and nano Fe(OH)3 as template. Optimal conditions for the catalytic oxygen reduction performance were determined as: the mass ratio of coal tar, nano Fe(OH)3 and KOH is 6:8:4; the pyrolysis temperature is 800 oC. SEM images show that the products have uniformly porous structure. TEM images demonstrate that the products are porous with foam shapes. HRTEM images further indicate that the products have formed several-layers 3D graphene structure, which are also supported by XRD and Raman data, and the pore size mainly distributes in 10 ~ 40 nm. XRD data show that the materials have a certain degree of graphitization. XPS spectra indicate that nano Fe(OH)3 template is washed out with no iron being detected and C element content is about 88.7% mainly comprising C—C bond. BET results demonstrate that the specific surface area is 2040 m2•g-1, the pore size distribution concentrates in 10 ~ 40 nm which is consistent with the results obtained by HRTEM. Electrochemical performances were tested in 0.1 mol•L-1 KOH, the initial reduction potential is 0 V (vs. Hg/HgO ) and the electron transfer number is 3.58. Such low-cost, good performance material is potentially useful for oxygen reduction catalyst.

/
| 〈 |
|
〉 |