

3, 4-乙烯二氧噻吩单体用作锂离子电池安全性改善添加剂的研究
收稿日期: 2015-12-29
修回日期: 2016-01-19
网络出版日期: 2016-01-25
基金资助
国家自然科学基金项目(No. 21373154)资助
3, 4-Ethylenedioxythiophene Monomer as Safety-Enhancing Additive for Lithium Ion Batteries
Received date: 2015-12-29
Revised date: 2016-01-19
Online published: 2016-01-25
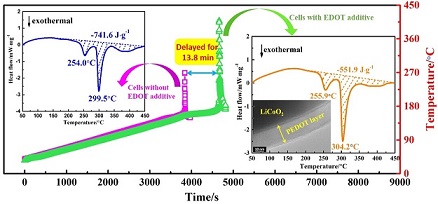
安全性是制约锂离子电池向电动汽车领域应用拓展的主要障碍. 本工作提出了一种能够有效改善锂离子电池安全性的电解液添加剂-3,4-乙烯二氧噻吩单体(EDOT),研究了其在有机电解液中的电氧化聚合行为,以及对LiCoO2电极高温热行为和电池安全性、电化学性能的影响. 循环伏安(CV)和透射电镜(TEM)表征结果表明,单体添加剂能够在电池充电过程发生电氧化聚合,在正极表面形成一层聚(3,4-乙烯二氧噻吩)(PEDOT)导电聚合物膜;差示扫描量热(DSC)分析结果显示,PEDOT隔离了电解液与正极表面的直接接触,减少了过热条件下电解液在正极表面的分解放热. 安全性测试结果表明,在电解液中仅添加0.1%的EDOT单体,即可将电池在150 oC高温热冲击下发生热失控的时间推迟13.8分钟. 电化学性能测试结果表明,聚合产物良好的电子导电性能有效改善正极的电子传导能力,在一定程度上提高电池的倍率性能和循环稳定性,而容量、低温性能等基本不受影响,展示出良好的应用前景.
关键词: 锂离子电池; 安全性添加剂; 电氧化聚合; 导电聚合物; 3, 4-乙烯二氧噻吩
吉维肖 , 王 凤 , 钱江锋 , 曹余良 , 艾新平 , 杨汉西 . 3, 4-乙烯二氧噻吩单体用作锂离子电池安全性改善添加剂的研究[J]. 电化学, 2016 , 22(3) : 271 -277 . DOI: 10.13208/j.electrochem.151244
Safety concern is a major obstacle hindering the wide applications of large-capacity lithium ion batteries (LIBs) in electric vehicles. In this paper, a polymerizable monomer of 3, 4-ethylenedioxythiophene (EDOT) was proposed and tested as an electrolyte additive for enhancing the safety of LIBs. The electro-oxidative polymerization behaviors and influence of PEDOT additive on the thermal behavior of LiCoO2 cathode, as well as the safety performance and electrochemical properties of LiCoO2-based LIBs were investigated. The results from cyclic voltammetry (CV) and transmission electron microscope (TEM) characterizations indicated that the monomer additive can be electro-oxidatively polymerized to form a dense and uniform conductive polymer film (PEDOT) on the cathode surface during the first battery charging. The analysis results from differential scanning calorimetry (DSC) demonstrated that the heat released from the thermal decomposition of liquid electrolyte on the cathode surface was significantly reduced by 26 % due to the PEDOT barrier layer, which prevents electrolyte from direct contact with highly oxidative cathode. The safety tests revealed that even with a monomer content of only 0.1 wt% in liquid electrolyte, the thermal runaway onset time of LiCoO2-based pouch full cells could be delayed for 13.8 min under high temperature impact at 150 oC, representing a significantly enhanced safety. In addition, it is also found that the use of EDOT monomer as an electrolyte additive did not produce any negative influence on the normal charge-discharge performance of LIBs, showing a prospect for battery application.

/
| 〈 |
|
〉 |