

三元正极材料前驱体Ni1/3Co1/3Mn1/3(OH)2的连续合成与条件探究
收稿日期: 2015-11-29
修回日期: 2015-12-30
网络出版日期: 2022-01-07
基金资助
山东省自主创新专项(2013CXA03073)和山东省自然科学基金(No. 2016ZRD03001)资助
Continuous Synthesis and Condition Exploration of Precursor Ni1/3Co1/3Mn1/3(OH)2 Ternary Cathode Material
Received date: 2015-11-29
Revised date: 2015-12-30
Online published: 2022-01-07
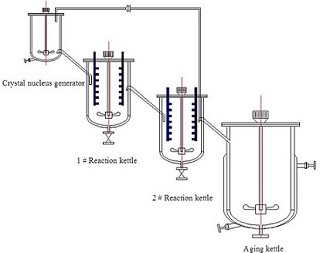
以硫酸锰、硫酸镍、硫酸钴为原材料、NaOH和氨水分别为沉淀剂和络合剂,采用共沉淀法制备三元正极材料前驱体Ni1/3Co1/3Mn1/3(OH)2. 探究了搅拌速度对造核颗粒形貌和晶核流量、氨水流量、浆料返流、搅拌桨对晶体结构、前驱体形貌、粒度及其粒度分布的影响. 物理表征结果表明,搅拌速度300 r•min-1时,生成的晶核聚集成球形或类球形,分散性好,颗粒粒径4~5 μm;在造核金属液流量0.4 L•h-1,生长金属液流量1.72 L•h-1,搅拌桨为推进式时,产物为单一相的β-Ni(OH)2层状结构,粒度D50为6~7 μm,振实密度≥2.0 g•cm-3,比表面积6~10 m2•g-1;电化学测试结果表明,在3.0~4.25 V电压范围内,0.2 C时,其首次放电容量为149.7 mAh•g-1,循环100次后,容量保持率为94.09 %;产物满足高端三元正极材料厂家需求. 多釜串联工艺简单有效,具有可行性,有望用于三元正极材料前驱体的规模生产.
关键词: 三元正极材料; 前驱体; Ni1/3Co1/3Mn1/3(OH)2; 多釜串联工艺
蒋志军 , 张亚莉 , 王乾 , 张慧 . 三元正极材料前驱体Ni1/3Co1/3Mn1/3(OH)2的连续合成与条件探究[J]. 电化学, 2016 , 22(5) : 528 -534 . DOI: 10.13208/j.electrochem.151128
Commercial LiNi1/3Co1/3Mn1/3(OH)2 ternary material is generally prepared by a combination of co-precipitation and solid state reaction method. The particle size distribution and morphology of Ni1/3Co1/3Mn1/3(OH)2 precursor have a great impact on the electrochemical performance of LiNi1/3Co1/3Mn1/3O2. In this work, the precursor Ni1/3Co1/3Mn1/3(OH)2 ternary cathode material was prepared by co-precipitation method with MnSO4, NiSO4, and CoSO4 as raw materials, NaOH as a precipitating agent and NH3•H2O as a complexing agent through continuous stirred-tank reactor series (CSTRs). The effects of stirring speed on the morphology of the nuclear particles, flow quantities of crystal nuclei and ammonia, slurry regurgitation, agitator blade structure on the morphology, crystalline structure, particle size and particle size distribution of the precursor were explored. Physical characterization results showed that the primary particles of the crystal nucleus agglomerate formed secondary particles with the particle size of 4 ~ 5 μm, and the well distributed particles were near spherical at the stirring rate of 300 r·min-1. When the pusher type agitator was used, the flow quantities of crystal nucleation and crystal growth metal liquids were 0.4 L•h-1 and 1.72 L•h-1, respectively, the product exhibited a single phase of β-Ni(OH)2 with a layered structure. The particle size (D50) of 6 ~ 7 μm, the tap density of ≥ 2.0 g•cm-3, and the BET surface area of 6 ~ 10 m2•g-1,were also obtained. The electrochemical test results revealed that the initial discharge capacity of LiNi1/3Co1/3Mn1/3O2 reached 149.7 mAh•g-1 and the capacity retention was 94.09% after 100 cycles in the voltage range of 3.0 ~ 4.25 V at 0.2 C. The product could meet demands of high-end ternary cathode material manufacturers. The CSTRs method is simple, effective and applicable. Therefore, it can be potentially used for a large scale preparation of ternary material precursors.

Key words: Ternary cathode material; Precursor; Ni1/3Co1/3Mn1/3(OH)2; CSTRs
/
| 〈 |
|
〉 |