

利用均相沉淀剂和形貌导向剂水热一步合成层状镍钴氢氧化物
Hydrothermal one-step synthesis of layered nickel/cobalt double hydroxide with using shape-directing agent and homogeneous precipitating agent
Received date: 2015-11-19
Revised date: 2015-12-22
Online published: 2015-12-25
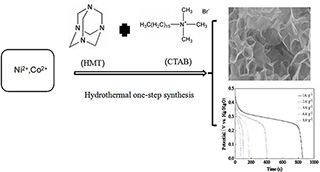
本文利用在反应过程中同时添加均相沉淀剂六次甲基四胺和形貌导向剂十六烷基三甲基溴化铵,结合水热反应的方法一步合成了镍钴氢氧化物. 随着六次甲基四胺的水解,层状镍钴氢氧化物可以被合成而且避免了额外碱源的使用;同时,由于反应过程中十六烷基三甲基溴化铵参与的孔径调节,合成出来的镍钴氢氧化物具有可控的介孔尺寸13.4 nm以及较大的比表面积93.6 m2 •g-1. X射线衍射图谱表明合成出来的镍钴氢氧化物构型是α-Ni(OH)2-β-Co(OH)2. 扫描电镜表明合成出来的镍钴氢氧化物具有层状的结构. 正是因为层状介孔结构的存在,合成出来的镍钴氢氧化物在1 A•g-1电流密度下,比电容可以高达1902 F•g-1;即使电流密度提高到8 A•g-1,镍钴氢氧化物的比电容仍然可以保持在1250 F•g-1.
关键词: 层状材料;十六烷基三甲基溴化铵; 镍钴氢氧化物;电化学性能
朱立伟* , 阎云海 , 贾慧 , 梁润芬 . 利用均相沉淀剂和形貌导向剂水热一步合成层状镍钴氢氧化物[J]. 电化学, 2016 , 22(4) : 412 -416 . DOI: 10.13208/j.electrochem.151119
Nickel/cobalt double hydroxide (Ni/Co-DH) has been prepared by facile hydrothermal one-step synthesis method using hexamethylene tetramine (HMT) as a homogeneous precipitating agent and cetyltrimethylammonium bromide (CTAB) as a shape-directing agent. With the hydrolysis of HMT and the use of CTAB, the Ni/Co-DH could be synthesized without additional alkali source, and in the meantime, the Ni/Co-DH exhibited a controlled mesopore size of 13.4 nm and a large BET surface area of 93.6 m2 •g-1 . The X-ray diffraction (XRD) pattern displays that the Ni/Co-DH existed as a mixture of α-Ni(OH)2-β-Co(OH)2. The scanning electron microscope (SEM) image shows that the as-prepared Ni/Co-DH took on a layered structure, which leads to a maximum specific capacitance of 1902 F•g-1 at a current density of 1 A•g-1. The specific capacitance remained 1250 F•g-1 when the current density increased to 8 A•g-1.

[1] Wang G P, Zhang L, Zhang J J.A review of electrode materials for electrochemical supercapacitors [J]. Chemical Society Reviews. 2012, 41:797-828 .
[2] Gao Z, Yang W L, Wang J, Song N N, et al. Flexible all-solid-state hierarchical NiCo2O4/porous graphene paper asymmetric supercapacitors with an exceptional combination of electrochemical properties [J]. Nano Energy. 2015,13: 306-317 .
[3] Hu ZA, Xie Y L, Wang Y X, et al. Synthesis and electrochemical characterization of mesoporous CoxNi1−x layered double hydroxides as electrode materials for supercapacitors [J]. Electrochimica Acta.2009,54:2737-2741.
[4] Yan X Y, Tong X L, Wang J, et al. Synthesis of mesoporous NiO nanoflake array and its enhanced electrochemical performance for supercapacitor application [J]. Journal of Alloys and Compounds. 2014,593: 184-189.
[5] Yan T, Li R Y, Yang T T,et al. Nickel/cobalt layered double hydroxide hollow microspheres with hydrangea-like morphology for high-performance supercapacitors [J]. Electrochimica Acta. 2015,152:530-537.
[6] Yan L, Li R Y, Li Z J, et al. Three-dimensional activated reduced graphene oxide nanocup/nickel aluminum layered double hydroxides composite with super high electrochemical and capacitance performances [J]. Electrochimica Acta. 2013,95:146-154.
[7] Dong S, Dao A Q, Zheng B J, et al. One-step electrochemical synthesis of three-dimensional graphene foam loaded nickel–cobalt hydroxides nanoflakes and its electrochemical properties [J].Electrochimica Acta. 2015,152:195-201.
[8] Deng W, Lan W, Sun Y R, et al. Porous CoO nanostructures grown on three-dimension graphene foams for supercapacitors electrodes [J] . Applied Surface Science. 2014,305:433-438.
[9] Zheng Q Y, Zhang X Y, Shen Y M. Construction of hierarchical porous NiCo2O4 films composed of nanowalls as cathode materials for high-performance supercapacitor [J]. Materials Research Bulletin.2015,64:401-404.
[10] Gupta V, Gupta S, Miura N. Al-substituted α-cobalt hydroxide synthesized by potentiostatic deposition method as an electrode material for redox-supercapacitors [J]. Journal of Power Sources. 2008,177: 685-689 .
[11] Pal T, Sau T K, Jana N R. Reversible formation and dissolution of silver nanoparticles in aqueous surfactant media [J]. Langmuir. 1997,13:1481-1485.
[12] Al-Thabait SAi, Obaid A Y, Hussain S,et al. Shape-directing role of cetyltrimethylammonium bromide on the morphology of extracellular synthesis of silver nanoparticles [J]. Arabian Journal of Chemistry. 2015,8: 538-544.
[13] Talebi-Esfandarani M., Savadogo O. Enhancement of electrochemical properties of platinum doped LiFePO4/C cathode material synthesized using hydrothermal method [J]. Solid State Ionics. 2012,61(4):81-86.
[14] Sierra-Fernandez A, Gomez-Villalba L.S, Milosevic O, Fort R, et al. Synthesis and morpho-structural characterization of nanostructured magnesium hydroxide obtained by a hydrothermal method [J]. Ceramics International. 2014,40:12285-12292.
[15] Xu M, Chen Z Y , Li L J ,et al. Highly crystalline alumina surface coating from hydrolysis of
aluminum isopropoxide on lithium-rich layered oxide [J].Journal of Power Sources, 2015,281:444-454.
[16] Yan T, Zhu H Y, Li R Y, et al. Microwave synthesis of nickel/cobalt double hydroxide ultrathin flowerclusters with three-dimensional structures for high-performance supercapacitors[J]. Electrochimica Acta. 2013,111: 71-79.
/
| 〈 |
|
〉 |