

不同锂盐对超支化/梳状复合型聚合物电解质的性能影响研究
收稿日期: 2015-10-13
修回日期: 2015-12-07
网络出版日期: 2016-01-04
基金资助
国家自然科学基金项目(No. 51473186)和广州市科技计划项目(No. 201508010052)资助
Effects of Lithium Salts on the Properties of Hyperbrandched/Comb-like Composite Polymer Electrolytes
Received date: 2015-10-13
Revised date: 2015-12-07
Online published: 2016-01-04
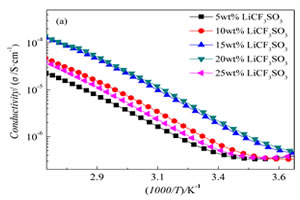
利用PVA侧链上的羟基的化学活性, 采用超支化聚胺-酯对改性纳米SiO2和PVA接枝改性, 并加入不同锂盐,制备了SiO2-g-HBPAE/PVA-g-HBPAE超支化/梳状复合型聚合物电解质, 利用SEM观察了纳米粒子在基体中的分散情况, 采用DSC、拉伸实验以及介电谱研究了锂盐种类及添加量对复合体系性能的影响. 结果表明, 超支化接枝改善了SiO2和基体的界面相容性; 磺酸类锂盐在复合材料中表现出自增塑现象, 材料的玻璃化转变温度(Tg)大幅度下降; LiClO4在基体中的离解能力强于LiCF3SO3和 LiN(SO3CF3)2; 当LiCF3SO3添加量为20 %(by mass, 下文同)时, 聚合物电解质的室温电导率达到最大值2.58×10-6 S•cm-1.
户献雷 , 梁晓旭 , 章明秋 , 张若昕 , 张利萍 , 阮文红 . 不同锂盐对超支化/梳状复合型聚合物电解质的性能影响研究[J]. 电化学, 2016 , 22(5) : 535 -541 . DOI: 10.13208/j.electrochem.151013
Based on the chemical characteristics of the hydroxyl group of PVA side-chain, the hyperbrandched poly(amine-ester) (HBPAE) was used to hypergraftingly pretreated nano-silica (SiO2) and polyving akohol (PVA). And different lithium salts were added to fabricate the SiO2-g-HBPAE/PVA-g-HBPAE hyperbrandched/comb-like composite polymer electrolytes (CPEs). The dispersion of nanoparticles in matrix was observed by SEM. The effects of different lithium salts on the properties of CPEs were studied by DSC, tensile test and dielectric spectra. The results showed that the hypergrafting method improved the interphase compatibility between SiO2 and matrix. Sulfonic acid type lithium salts accelerated self-plasticizing with the Tg values being decreased. The LiClO4 manifested stronger solubility than LiCF3SO3 and LiN(SO3CF3)2 in the polymer matrices. The ionic conductivity of the polymer electrolytes reached the maximum value of 2.58×10-6 S·cm-1 after the addition of 20% LiCF3SO3 at room temperature.

/
| 〈 |
|
〉 |