

基于对羟基苯硼酸为前驱体的金纳米粒子修饰电极电化学检测过氧化氢
收稿日期: 2015-09-08
修回日期: 2015-11-08
网络出版日期: 2015-11-16
基金资助
吉林省教育厅项目(No. 2013318,No. 2013372)、吉林省社会科学项目(No. 2012B260)资助
Electrochemical Detection of Hydrogen Peroxide at AuNPs Modified Electrode Using p-Hydroxyphenylboronic Acid as a Precursor
Received date: 2015-09-08
Revised date: 2015-11-08
Online published: 2015-11-16
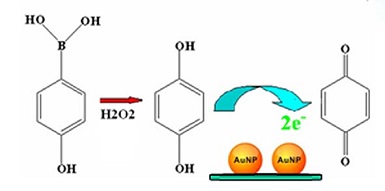
以对羟基苯硼酸为前驱体,利用H2O2可以定量氧化对羟基苯硼酸产生对羟基苯酚的原理,以反应产物对羟基苯酚为电化学信号物质,结合金纳米粒子修饰玻碳电极(AuNPs/GCE),发展了一种间接检测H2O2的电化学方法. 由于AuNPs/GCE具有有效电子传递性能和比表面积大等优点,对硼酸氧化产物具有较高的催化活性,因此在含1.0 mmol•L-1对羟基苯硼酸的0.1 mol•L-1 pH 7.5 PBS中,AuNPs/GCE可以检测到1.0 ~ 1.0 × 103 μmol•L-1的H2O2,检测限为0.5 μmol•L-1. 同时,该方法具有良好的选择性和重现性,且操作简单、速度快、价格低廉,非常适用于实际样品中H2O2含量的测定.
王春燕* , 刘晓秋 , 戚颖欣 . 基于对羟基苯硼酸为前驱体的金纳米粒子修饰电极电化学检测过氧化氢[J]. 电化学, 2016 , 22(1) : 88 -93 . DOI: 10.13208/j.electrochem.150908
We developed a new method for electrochemical detection of hydrogen peroxide (H2O2) based on boronate oxidation of p-hydroxyphenylboronic acid. This method using p-aminophenol which is produced from the reaction of H2O2 and p-aminophenylboronic acid as a well electrochemical probe, combined with a gold nanoparticles (AuNPs) modified electrode for an indirect detection of H2O2. Because of the large surface area and enhanced electrocatalytic behavior by the AuNPs modified electrode, the detection sensitivity was improved. The method could detect H2O2 in the concentration range of 1 ~ 100 μmol•L-1 and 0.1 ~ 1 mmol•L-1 in 0.1 mol•L-1 pH 7.5 PBS containing 1.0 mmol•L-1 p-hydroxyphenylboronic acid. The low detection limit was 0.5 μmol•L-1. The proposed method had good selectivity and stability. Moreover, the method was quick, simple and cheap, which has potential application in real samples analysis.

/
| 〈 |
|
〉 |