

基于花状铂纳米颗粒构建的电致化学发光免疫传感器用于检测载脂蛋白A1
收稿日期: 2015-09-14
修回日期: 2015-11-10
网络出版日期: 2015-11-18
基金资助
国家自然科学基金项目(No. 21575116)资助
Electrochemiluminescence Immunosensor Based on Platinum Nanoparticles for the Determination of Apolipoprotein A1
Received date: 2015-09-14
Revised date: 2015-11-10
Online published: 2015-11-18
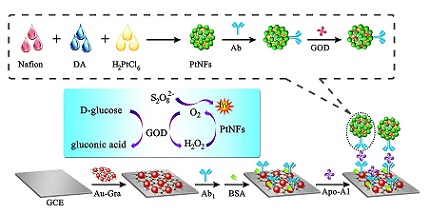
采用一锅合成法制备了新型的具有大比表面积的花状铂纳米颗粒(PtNFs),并构建了一个高灵敏电致化学发光(ECL)免疫传感器用于检测载脂蛋白A1(Apo-A1). 该PtNFs用于吸附二抗(anti-Apo-A1),并用葡糖糖氧化酶(GOD)封闭其表面的非特异性位点,最终制备了PtNFs@anti-Apo-A1@GOD信号探针. 当Apo-A1存在时,通过夹心免疫反应将制备的信号探针捕获于电极表面,并将所制得的电极置于含有葡萄糖的过硫酸根底液中检测. GOD催化葡萄糖产生H2O2,H2O2在PtNFs的催化下分解并在电极表面原位产生O2,所产生的O2能够催化过硫酸根-氧气体系的电致化学发光反应,放大发光信号,提高检测灵敏度. 该传感器在0.1 ng•mL-1 ~ 100 ng•mL-1范围内对Apo-A1有良好的线性响应,检测下限达到0.03 ng•mL-1,有望应用于临床分析诊断.
关键词: 花状铂纳米颗粒; 电致化学发光免疫传感器; 载脂蛋白A1
廖 妮 , 卓 颖 , 袁 若 . 基于花状铂纳米颗粒构建的电致化学发光免疫传感器用于检测载脂蛋白A1[J]. 电化学, 2016 , 22(3) : 299 -305 . DOI: 10.13208/j.electrochem.150914
In this paper, a novel electrochemiluminescence (ECL) immunosensor for the detection of apolipoprotein A1 was constructed based on flower-like platinum nanoparticles (PtNFs) via a one-pot chemical synthesis method. The PtNFs was used to immobilize the secondary antibody and enzyme (GOD). Then the prepared bioconjugates were introduced onto the electrode via sandwich immunoreactions. Accordingly, the ECL luminophore peroxydisulfate (S2O82- ) was presented in the working buffer solution containing an appropriate amount of glucose. Through the ECL responses of S2O82- and O2, a dramatically amplified ECL signal was obtained for the reason that hydrogen peroxide (H2O2) produced by GOD to glucose was subsequently catalyzed by PtNFs to in situ generate O2. The present immunosensor showed a wide linear range of 0.1 ng•mL-1 to 100 ng•mL-1, with a low detection limit of 0.03 ng•mL-1 for detecting Apo-A1. This new signal amplification strategy for preparation of ECL immunosensor could be easily realized and has potential application in ultrasensitive bioassays.

/
| 〈 |
|
〉 |