

阻燃剂对镍钴锰三元材料体系电化学性能的影响
收稿日期: 2015-09-24
修回日期: 2015-11-10
网络出版日期: 2015-12-02
基金资助
国家自然科学基金项目(No. 21203040)、中国博士后面上项目(No. 2014M561357)、浙江省博士后科研项目择优资助基金(No. zj140288)及黑龙江省自然科学基金项目(No. B201201)资助
Effect of Flame Retardant on Electrochemical Performances of Lithium Ion Battery with LiNi0.4Co0.2Mn0.4O2 as a Positive Electrode Material
Received date: 2015-09-24
Revised date: 2015-11-10
Online published: 2015-12-02
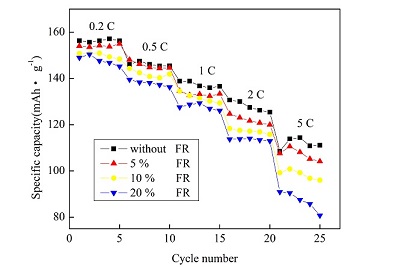
测试了不同浓度的电解液阻燃添加剂对镍钴锰三元材料(LiNi0.4Co0.2Mn0.4O2)作为正极的锂离子电池电化学性能的影响. 实验结果表明,当阻燃剂浓度增加时,电池的放电容量下降,电化学反应电阻和锂离子扩散阻力都有所增加,但加入阻燃添加剂的锂离子电池,当充放电的电流相对比较小的时候,循环性能相比于不含阻燃剂的有所提高,循环稳定性得到了改善. 在0.5C倍率电流下,不含阻燃剂时容量保持率为89%,而当阻燃剂含量增至10%后保持率达到94.21%. 当充放电电流为1C时,未使用阻燃剂时容量保持率约为92.22%,当阻燃剂的浓度为10%时容量保持率为93.01%. 在2C倍率下,不含阻燃剂时容量保持率为87.92%. 阻燃剂浓度为10%时,容量保持率有所提升,达到92.16%. 与基础电解液相比,选用含有10%阻燃剂的电解液可使容量保持率提高5%左右,相比于其他浓度,包含10%阻燃剂的电池循环性能也最为稳定.
关键词: 锂离子电池; LiNi0.4Co0.2Mn0.4O2; 电解液; 阻燃添加剂; 电化学性能
吕艳卓 , 葛永先 , 王振波 , 柯 克 . 阻燃剂对镍钴锰三元材料体系电化学性能的影响[J]. 电化学, 2016 , 22(1) : 70 -74 . DOI: 10.13208/j.electrochem.150925
The effect of flame retardant on the electrochemical performances of lithium ion battery by using LiNi0.4Co0.2Mn0.4O2 as a positive material was studied by measuring the first charge-discharge, cycling, circulation, and AC impedance curves. The experimental data indicated that the discharge capacity of the battery decreased, while the charge transfer resistance and the lithium ion diffusion resistance increased with the increases in the concentrations of the flame retardant in the electrolyte solutions. Furthermore, the stability of the battery at small discharge ratios and the cyclic performance were also improved by the addition of flame retardant. The capacitance retention values went up from 89% without the addition of flame retardant to 94.21% with the addition of 10% flame retardant at the discharge ratio of 0.5C, from 92.22% to 93.01% at 1C, and from 87.92% to 92.16% at 2C. The best cycle stability of the battery was achieved with the addition of 10% flame retardant.

/
| 〈 |
|
〉 |