

温度对空间用氢镍电池电化学性能的影响
Temperature Effects on Properties of Aerospace Nickel Hydrogen Cells
Received date: 2015-07-20
Revised date: 2015-11-09
Online published: 2015-11-11
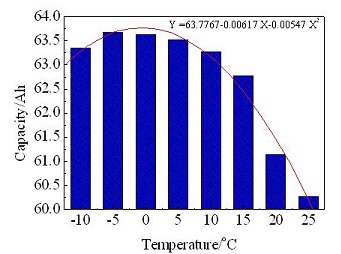
以60 Ah氢镍电池为研究对象,研究了温度对电池电性能的影响. 结果表明,电池的放电容量、过充电率随着温度均呈先升后降趋势,最高放电容量可达63.68 Ah(-5 oC),电池的适合涓流值及3天自放电率随着温度的升高呈增加趋势,电池的放电容量、过充电率、适合涓流值和自放电率与环境温度之间有近似的代数公式变化关系. -10 oC、80%放电深度(DOD)条件下循环3000次后,电池电性能无明显衰降;25 oC下循环550次,放电电压跌至0.8 V,电池失效. 结合相关参考文献结果及EIS试验分析可知,25 oC下电池循环性能迅速失效主要是由于高温下镍电极更易析氧和发生极板腐蚀,以及高温下镍极板更易粉化所致.
张海昌* , 刁玉琦 , 明文成 , 任学颖 , 丁 飞 . 温度对空间用氢镍电池电化学性能的影响[J]. 电化学, 2016 , 22(1) : 49 -56 . DOI: 10.13208/j.electrochem.150720
This has been done using the 60 Ah nickel-hydrogen cell to investigate the temperature effects on properties of aerospace nickel hydrogen cells. The charge-discharge, trickle charge, overcharge, self-discharge and cycle life tests were carried out at different temperatures. The results show that the discharge capacity and overcharge rate were increased first, and then decreased with the raising temperature. On the other hand, the trickle charge value and the 3 days self-discharge rate were raised with increasing temperature. When the temperature was -5 oC, the discharge capacity of cell reached the maximum discharge capacity of 63.68 Ah. Based on the test results, the approximate functions among the discharge capacity, trickle charge rate, overcharge rate, self-discharge rate and temperature can be described. The battery lifetime results at 80% DOD and -15 oC indicate no apparent battery deterioration even after 3000 cycles. When the lifetime operating at 80% DOD and 25 oC, the cut-off voltage for end discharge of the cell dropped to 0.8 V and the battery failed. Based on the related references and EIS results, it was concluded that the temperature increase resulted in oxygen electrode potential drop, and the early start of oxygen evolution at charge and nickel plate pulverized, which markedly speeds up the cell performance degradation at life cycling.

/
| 〈 |
|
〉 |