

对磺酸基苯偶氮杯[4]芳烃电化学行为研究
收稿日期: 2015-06-04
修回日期: 2015-10-14
网络出版日期: 2015-10-19
基金资助
教育部科学技术研究重点项目(No. 209031)资助
An Electrochemical Investigation of p-sulfophenylazo Calix[4]arene in a Buffer Solution
Received date: 2015-06-04
Revised date: 2015-10-14
Online published: 2015-10-19
以杯[4]芳烃和对氨基苯磺酸为原料,经重氮化-偶联反应合成了对磺酸基苯偶氮杯[4]芳烃,并使用紫外吸收光谱、红外吸收光谱、核磁共振等技术对其进行表征分析. 首次以CH3COOH-CH3COONa为缓冲溶液,使用循环伏安法研究对磺酸基苯偶氮杯[4]芳烃的电化学行为. 结果表明,当扫描电位在-0.5 ~ 1 V时,有1对氧化还原峰,其中氧化峰电位为0.302 V,还原峰电位为-0.003 V,且峰电流与峰电位均与扫描速率呈线性关系,推测该峰的形成受扩散控制影响,反应为动力学准一级可逆反应. 进一步利用多种电化学手段研究该电极反应,并求得动力学参数,反应活化能为14.84 kJ•mol-1.
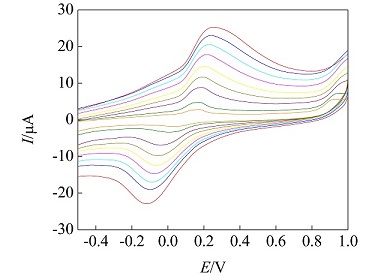
关键词: 对磺酸基苯偶氮杯[4]芳烃; 循环伏安法; 玻碳电极; 电化学行为
杨 星 , 陈 平* . 对磺酸基苯偶氮杯[4]芳烃电化学行为研究[J]. 电化学, 2016 , 22(1) : 37 -42 . DOI: 10.13208/j.electrochem.150604
The p-sulfophenylazo calix[4]arene was synthesized through the diazotization-coupling reaction between calix[4]arene and p-aminobenzene sulfonic acid. The UV-Vis spectroscopy, Infrared spectroscopy, and nuclear magnetic resonance (NMR) were used to characterize the as-prepared p-sulfophenylazo calix[4]arene. The electrochemical behaviors of p-sulfophenylazo calix[4]arene were investigated by cyclic voltammetry and chronoamperometry using CH3COOH-CH3COONa as a buffer solution. The result shows that the p-sulfophenylazo calix[4]arene exhibited a pair of redox peaks in a scan potential region of -0.5 ~ 1 V. The oxidation and reduction peak potentials were 0.302 V and -0.003 V, respectively, and changed with the scanning rates. In addition, the electrode reaction was studied by various electrochemical methods, and the kinetic parameters have also been obtained. The activation energy was found to be 14.84 kJ•mol-1.

/
| 〈 |
|
〉 |