

铁、氮掺杂石墨烯/碳黑复合材料的制备及氧还原电催化性能
收稿日期: 2015-12-08
修回日期: 2016-01-04
网络出版日期: 2016-01-06
基金资助
国家自然科学基金项目(No. 21373175)资助
Synthesis of Fe, N-doped Graphene/Carbon Black Composite with High Catalytic Activity for Oxygen Reduction Reaction
Received date: 2015-12-08
Revised date: 2016-01-04
Online published: 2016-01-06
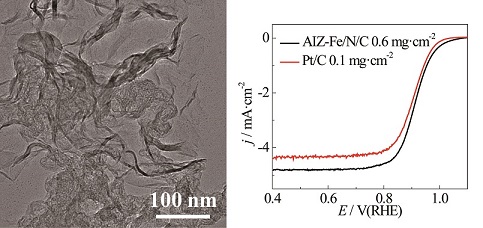
以高含氮量的2-氨基咪唑为氮源,三氯化铁为铁源,高比表面积的KJ600碳黑为载体,通过水热法制得氨基咪唑聚合物前驱体,再经二次高温热处理,制得石墨烯/碳黑复合材料. 透射电镜表征显示该材料为石墨烯纳米片与碳黑颗粒的复合结构. BET表征表明这是一种多孔结构,具有很高的比表面积(882 m2•g-1),这有利于暴露更多活性位点,并促进传质. XRD证实催化剂中存在石墨烯,且石墨烯结构是在第一次热处理过程中形成的. 电化学测试表明,该催化剂在酸性和碱性介质中都具有很高的氧还原电催化活性和低H2O2产率,并且在碱性介质中对甲醇小分子的抗毒化性能明显优于商业Pt/C催化剂,展示出在实际燃料电池系统中的应用潜力.
关键词: 2-氨基咪唑; N掺杂石墨烯; 氧气还原反应; 石墨烯/碳黑复合结构
陈 驰 , 周志有* , 张新胜 , 孙世刚 . 铁、氮掺杂石墨烯/碳黑复合材料的制备及氧还原电催化性能[J]. 电化学, 2016 , 22(1) : 25 -31 . DOI: 10.13208/j.electrochem.150849
Oxygen reduction reaction (ORR) is a bottleneck for improving the efficiency of proton-exchange membrane fuel cells as a cathode reaction due to its sluggish kinetics. The exploitation of low cost and high performance non-precious metal catalysts such as Fe/N/C based materials toward ORR has attracted extensive attentions. In this work, the Fe, N-doped graphene nanosheets/carbon black composite was prepared by hydrothermal polymerization and followed by a twice-heat-treatment procedure using 2-aminoimidazole as an N precursor, FeCl3 as an Fe precursor and KJ600 carbon black as a support. The TEM images revealed that the graphene nanosheets were separated by carbon black nanoparticles to form a robust composite architecture. This composite structure can provide high surface area and porous structure, facilitating the exposure of active sites and the mass transfer of O2. The XRD patterns proved the existence of graphene nanosheets formed during the first heat treatment. The obtained AIZ-Fe/N/C catalyst exhibited high ORR activity and low H2O2 yield in an alkaline medium. Its methanol resistance was much better than that of commercial Pt/C catalyst. Furthermore, the ORR activity in an acid medium was also impressive. These results demonstrated that the AIZ-Fe/N/C catalyst is a promising candidate to replace Pt-based catalysts as a cathode catalyst in fuel cells.

/
| 〈 |
|
〉 |