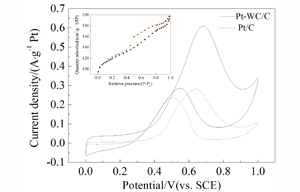
以酚醛树脂作为碳源,采用嵌段共聚物模板法一步制备新型有序介孔碳化钨/碳(WC/C)纳米颗粒. WC/C颗粒的比表面积为414 m2·g-1,表面的平均孔径约为38 nm,处于介孔范围内(2 ~ 50 nm). 通过调节树脂预聚时间以及碳化温度等条件制备出结构形貌较优的WC/C复合材料,并探讨了材料形成机理. 使用X射线衍射、扫描电镜、透射电镜及氮气吸脱附等方法表征了复合材料的结构. 将贵金属铂负载于WC/C表面制备得新电催化材料Pt-WC/C,使用循环伏安法和计时电流法对Pt-WC/C复合材料的电化学性能进行检测,并与商用碳载铂(Pt/C)材料进行对比. 测试结果发现,Pt-WC/C对甲醇的电催化活性以及稳定性等方面都表现出优于商用Pt/C材料的活性,这主要归功于碳化钨高度分散于碳表面.
At present, one of the major hurdles for commercialization of system with direct methanol fuel cell (DMFC) is still the requirement of a significant amount of platinum (Pt) catalyst to achieve an acceptable power density. Pt and Pt-based metals are expensive due to limited supplies. In order to find the catalyst alternative to the Pt metal, we selected tungsten carbide (WC) and its composites as the study object. We synthesized WC with carbon materials as the suitable support to modify the electronic structure and to increase the specific surface area of WC. In this study, tungsten carbide/carbon (WC/C) was prepared by block copolymer template synthesis method using resorcinol-formaldehyde resin (RF) as a carbon source. These novel, ordered, mesoporous WC/C nanocomposites showed high surface areas (414 m2·g-1) and appropriate pore-size (about 38 nm). The experimental conditions including the stirring time of phenol formaldehyde polymer and carburization temperature were systematically studied. The results indicated that the composites had optimized crystal phases and ordered arrangement of pores structures. The characteristics of WC/C composites were determined by X-ray diffraction, scanning electron microscopy, transmission electron microscopy and Brunauer-Emmet-Teller gas adsorption. The Pt nanoparticles were uniformly distributed on WC/C and a new electrocatalyst of Pt-WC/C was prepared by microwave-assisted (MW) polyol method. The electro-catalytic performances of the as-prepared Pt-WC/C and commercial carbon-supported Pt (Pt/C) toward methanol oxidation were compared by cyclic voltammetry and chronoamperometry. It was found that the Pt-WC/C exhibited higher catalytic activity for methanol oxidation than commercial Pt/C catalyst. Especially, the Pt-WC/C achieved the long-term stability which was attributed to the high dispersion of WC with smaller sizes on the surface of carbon.