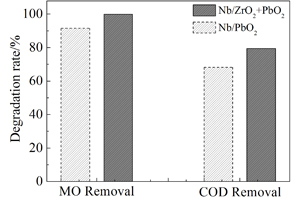
本文利用电沉积制得ZrO2掺杂铌基二氧化铅电极,通过扫描电镜(SEM)和X射线衍射(XRD)观察表征了Nb/ZrO2+PbO2电极表面形貌特征及组成. 用线性伏安曲线、电化学交流阻抗和循环伏安曲线测试了Nb/ZrO2+PbO2电极电化学性能,及其甲基橙(MO)的降解电催化效果. 结果表明,ZrO2掺杂使Nb/PbO2电极表面更致密、粗糙,结晶尺寸更小,增大了电极比表面积,提高了电极电催化活性. Nb/ZrO2+PbO2电极活性层主要由β-PbO2和少量的α-PbO2以及部分ZrO2共沉积而成. 有较高的析氧电位、吸附电容和较低的电荷转移电阻,其甲基橙(MO)降解电催化活性更佳,降解过程受扩散控制.
The zirconium oxide (ZrO2)-doped niobium based lead oxide (Nb/PbO2) electrode was prepared by electrodeposition. The microstructures and electrochemical properties of Nb/ZrO2+PbO2 electrode were investigated using scanning electron microscopy (SEM), X-ray diffraction (XRD), linear sweep voltammetry, electrochemical impedance spectroscopy and cyclic voltammotry. In addition, the Nb/PbO2 electrode was used as an anode for the electrochemical degradation of methyl orange (MO). Results revealed that the ZrO2 doping made the Nb/PbO2 electrode surface denser and rougher with smaller sized crystal particles, which increased the specific surface area and enhanced the electrocatalytic activity of the electrode. The Nb/ZrO2+PbO2 electrode was mainly composed of β-PbO2 with a small amount of α-PbO2 and ZrO2 formed by codeposition. Accordingly, the Nb/ZrO2+PbO2 electrode possessed higher oxygen-evolution potential, larger adsorption capacity and lower charge-transfer resistance, as well as higher electrocatalytic activity in the degradation of organic materials than the Nb/PbO2 electrode. The MO degradation processes were irreversible and controlled by diffusion.