

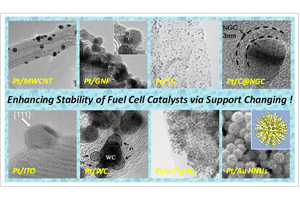
通过载体提高燃料电池催化剂的稳定性
收稿日期: 2014-11-25
修回日期: 2015-04-04
网络出版日期: 2015-06-28
基金资助
国家自然科学基金项目(No. 20936008,No. 21176271,No. 21276291,No. 21376283,No. 21376284)资助
Enhancing Stability of PEM Fuel Cell Catalysts via Support Changing
Received date: 2014-11-25
Revised date: 2015-04-04
Online published: 2015-06-28
谢小红 , 魏子栋 . 通过载体提高燃料电池催化剂的稳定性[J]. 电化学, 2015 , 21(3) : 221 -233 . DOI: 10.13208/j.electrochem.141052
/
| 〈 |
|
〉 |