

石墨烯包覆富锂层状材料的制备及其电化学性能
收稿日期: 2014-11-27
修回日期: 2015-03-26
网络出版日期: 2015-06-28
基金资助
国家重大研究计划纳米专项项目(No. 2011CB935903)资助
Synthesis of Graphene Wrapped Li-rich Layered metal Oxide and its Electrochemical Performance
Received date: 2014-11-27
Revised date: 2015-03-26
Online published: 2015-06-28
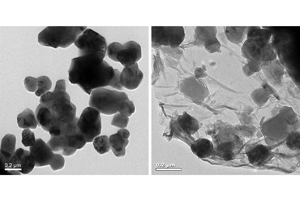
侯孟炎 , 王珂 , 董晓丽 , 夏永姚 . 石墨烯包覆富锂层状材料的制备及其电化学性能[J]. 电化学, 2015 , 21(3) : 195 -200 . DOI: 10.13208/j.electrochem.141053

/
| 〈 |
|
〉 |