

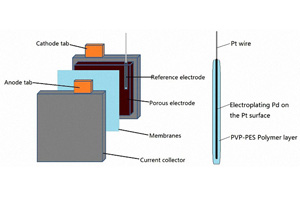
全钒液流电池铂氢微型参比电极体系的构筑与性能
收稿日期: 2014-12-09
修回日期: 2015-01-19
网络出版日期: 2015-04-23
A Platinum-Hydrogen Reference Micro-Electrode for All Vanadium Redox Flow Battery
Received date: 2014-12-09
Revised date: 2015-01-19
Online published: 2015-04-23
林志彬 , 董燕青 , 郑明森 , 董全峰 . 全钒液流电池铂氢微型参比电极体系的构筑与性能[J]. 电化学, 2015 , 21(2) : 162 -166 . DOI: 10.13208/j.electrochem.141209
/
| 〈 |
|
〉 |