

锂空气电池高容量长寿命Co3O4纳米空心球阴极催化剂
收稿日期: 2014-11-03
修回日期: 2015-02-01
网络出版日期: 2015-04-23
基金资助
基金委优青(No. 21422108)资助
Porous Co3O4 Hollow Nanospheres Cathode Catalyst for High-capacity and Long-cycle Li-Air Batteries
Received date: 2014-11-03
Revised date: 2015-02-01
Online published: 2015-04-23
刘通 , 李娜 , 刘清朝 , 张新波 . 锂空气电池高容量长寿命Co3O4纳米空心球阴极催化剂[J]. 电化学, 2015 , 21(2) : 157 -161 . DOI: 10.13208/j.electrochem.141049
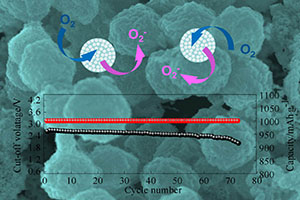
Key words: Co3O4 hollow spheres; lithium-air batteries; long life; hydrothermal
/
| 〈 |
|
〉 |