

CoAl2O4包覆LiNi1/3Co1/3Mn1/3O2的电化学性能
收稿日期: 2014-10-26
修回日期: 2014-12-22
网络出版日期: 2014-12-31
基金资助
福建省自然科学基金项目(NO. 2012J05028)资助
Electrochemical Performance of CoAl2O4-Coated LiNi1/3Co1/3Mn1/3O2
Received date: 2014-10-26
Revised date: 2014-12-22
Online published: 2014-12-31
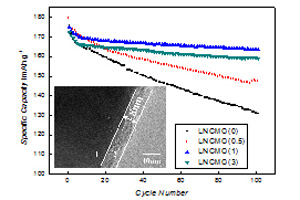
关键词: 锂离子电池; LiNi1/3Co1/3Mn1/3O2; 电化学性能; CoAl2O4包覆
蔡济钧 , 崔王君 , 李冰 , 余洋洋 , 赵金保 . CoAl2O4包覆LiNi1/3Co1/3Mn1/3O2的电化学性能[J]. 电化学, 2015 , 21(2) : 145 -151 . DOI: 10.13208/j.electrochem.141043

/
| 〈 |
|
〉 |