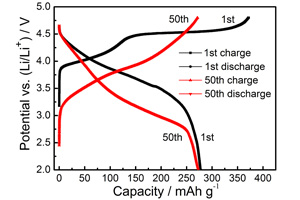
随着新能源如电动汽车、储能电站的蓬勃发展,人们对下一代高性能锂离子电池的能量密度、功率密度和循环寿命提出了更高的要求. 而富锂锰基正极材料xLi2MnO3·(1-x)LiMO2(0 < x < 1,M = Mn、Co、Ni…)具有可逆比容量高(240 ~ 280 mAh·g-1,2.0 ~ 4.8 V)、电化学性能较佳、成本较低等优点,已吸引了研究者的关注,有望成为下一代锂离子电池用正极材料. 本实验室采用固相法和溶胶-凝胶法制备不同的富锂锰基正极材料,其中,溶胶-凝胶法制得的Li[Li0.2Mn0.54Ni0.13Co0.13]O2电极首周期放电比容量277.3 mAh·g-1,50周期循环后容量272.8 mAh·g-1,容量保持率98.4%. 本文重点结合本实验室的研究工作,对新型富锂锰基正极材料xLi2MnO3·(1-x)LiMO2的结构、合成、电化学性能改性和充放电机理等进行总结与评述.
With rapid development of new energy industry like electric vehicles and energy storage station, these fields highly demand the next generation of high performance Li-ion battery systems with stronger energy density, higher power density, and longer cycling life. Lithium-rich Mn-based cathode materials, xLi2MnO3·(1-x)LiMO2(M=Mn, Co, Ni...), have become the hot topic and drawn attentions of scholars worldwide because of their high reversible capacity exceeding 240 mAh·g-1, excellent electrochemical properties, and low cost, which makes them most promising cathode material candidates for next Li-ion battery system. The cathode material Li[Li0.2Mn0.54Ni0.13Co0.13]O2 prepared in our laboratory shows high initial discharge capacity of 277.3 mAh·g-1 with retention of 98.4% after 50 cycles. Based on our previous works, we have introduced and reviewed the structures, preparation methods, and charge/discharge mechanisms of these lithium-rich Mn-based cathode materials xLi2MnO3·(1-x)LiMO2.