

Li3Fe2(PO4)3/C正极材料的电化学性能及其反应机理研究
收稿日期: 2014-11-03
修回日期: 2014-12-19
网络出版日期: 2014-12-25
基金资助
国家重点基础研究发展计划(No. 2011CB935903)、国家自然科学基金项目(No. 21233004,No. 21303147)和福建省自然科学基金项目(No. 2014J05019)资助
Electrochemical Performance and Reaction Mechanism of Li3Fe2(PO4)3/C Cathode Material
Received date: 2014-11-03
Revised date: 2014-12-19
Online published: 2014-12-25
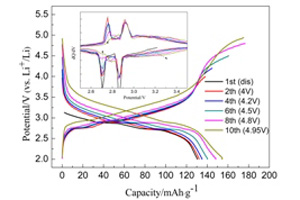
关键词: 锂离子电池; Li3Fe2(PO4)3/C; 电化学性能; 电化学原位XAFS
谢勇 , 钟贵明 , 龚正良 , 杨勇 . Li3Fe2(PO4)3/C正极材料的电化学性能及其反应机理研究[J]. 电化学, 2015 , 21(2) : 123 -129 . DOI: 10.13208/j.electrochem.141045

/
| 〈 |
|
〉 |