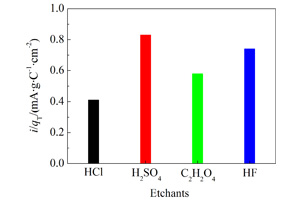
采用传统热分解法制得了不同刻蚀剂(HCl、H2SO4、C2H2O4和HF)处理的Ti基IrO2-MnO2纳米涂层阳极,使用场发射扫描电子显微镜(FESEM)、循环伏安(CV)及极化技术等观察和研究各纳米涂层阳极表面形貌及其电化学性能. 结果表明,与HCl和C2H2O4刻蚀剂相比,HF和H2SO4刻蚀的基底涂层表面IrO2纳米颗粒更为密集且尺寸更大;H2SO4刻蚀处理的Ti基IrO2-MnO2阳极电催化活性最佳,HF次之,C2H2O4再次之,HCl最差.
The IrO2-MnO2 anodic nano-coatings were prepared by the traditional thermal decomposition method on the different surfaces of Ti substrates pretreated by different etchants (HCl, H2SO4, C2H2O4 and HF). Surface morphologies and electrochemical performance of the Ti based IrO2-MnO2 anodic coatings were investigated by field emission scanning electron microscopy (FESEM), cyclic voltammetry (CV) and polarization techniques. Analysis showed that the denser IrO2 nano-particles with longer dimensions were obtained at the IrO2-MnO2 anodic coating surfaces on the Ti substrates etched by HF and H2SO4 as compared with those on the Ti substrates etched by HCl and C2H2O4. The decreasing order of electro-catalytic activities for the IrO2-MnO2 anodic nano-coatings on the Ti substrates etched by different etchants is H2SO4 > HF > C2H2O4 > HCl.
[1]Ye Z G, Meng H M, Chen D, et al. Structure and characteristics of Ti/IrO2(x)+MnO2(1-x) anode for oxygen evolution[J]. Solid State Sciences, 2008, 10(3): 346-354.
[2]Ye Z G, Meng H M, Sun D B. New degradation mechanism of Ti/IrO2+MnO2 anode for oxygen evolution in 0.5M H2SO4 solution[J]. Electrochimica Acta, 2008, 53(18): 5639-5643.
[3]Ye Z G, Meng H M, Sun D B. Electrochemical impedance spectroscopic (EIS) investigation of the oxygen evolution reaction mechanism of Ti/IrO2(x)+MnO2(1-x) electrodes in 0.5M H2SO4 solution[J]. Journal of Electroanalytical Chemistry, 2008, 621(1): 49-54.
[4]Zhou X L, Ye Z G, Hua X Z, et al. Electrocatalytic activity and stability of Ti/IrO2+MnO2 anode in 0.5 M NaCl solution[J]. Journal of Solid State Electrochemistry, 2010, 14(7): 1213-1219.
[5]Bai S J(白少金), Wei Z P(魏宗平), Wang X(王欣), et al. Preparation and performance of novel IrO2-Ta2O5 coated titanium anode with embedded nanoscale IrO2[J]. Transactions of Nonferrous Metals Society of China(中国有色金属学报), 2011, 21(3): 669-674.
[6]Xu H(徐浩), Yan W(延卫), You L(游莉). Effects of various acids treatment on the properties of titanium substrate[J]. Rare Metal Materials and Engineering(稀有金属材料与工程), 2011, 40(9): 1550-1554.
[7]Lamolle S F, Monjo M, Rubert M, et al. The effect of hydro?uoric acid treatment of titanium surface on nanostructural and chemical changes and the growth of MC3T3-E1 cells[J]. Biomaterials, 2009, 30(5): 736-742.
[8]Fóti G, Mousty C, Reid V, Comninellis C. Characterization of DSA type electrodes prepared by rapid thermal decomposition of the metal precursor[J]. Electrochimica Acta, 1998, 44(5): 813-818.
[9]Simon P, Gogotsi Y. Materials for electrochemical capacitors[J]. Nature Materials, 2008, 7(11): 845-854.
[10]Toupin M, Brousse T, Belanger D. Charge storage mechanism of MnO2 electrode used in aqueous electrochemical capacitor[J]. Chemistry of Materials, 2004, 6(16): 3184-3190.
[11]Spinolo G, Ardizzone S, Trasatti S. Surface Characterization of Co3O4 electrodes prepared by the sol-gel method[J]. Journal of Electroanalytical Chemistry, 1997, 423(1): 49-57.
[12]Yeo R S, Orehotsky J, Visscher W, et al. Ruthenium-based mixed oxides as electrocatalysts for oxygen evolution in acid electrolytes[J]. Journal of Electroanalytical Chemistry, 1981, 128(9): 1900-1904.
[13]Li Y J, Chang C C, Wen T C. A mixture design approach to thermally prepared Ir-Pt-Au ternary electrodes for oxygen reduction in alkaline solution[J]. Journal of Applied Electrochemistry, 1997, 27(2): 227
[14]Hu J M, Meng H M, Zhang J Q, et al. Effect of crystallite orientation of IrO2 rutile on the corrosion characteristics of IrO2+Ta2O5 oxide coatings[J]. Journal of Materials Science Letters, 2001, 20(14): 1353-1355.