

镁钙合金表面贻贝类吸附蛋白膜的NaIO4氧化处理及抗腐蚀性能
收稿日期: 2014-05-07
修回日期: 2014-05-19
网络出版日期: 2014-05-25
基金资助
国际科技合作与交流专项(No. 2014DFG52350)和国家自然科学基金(No. 21321062, No. 21203158)资助
NaIO4 Oxidation of Mussel Adhesive Protein Film and Its Corrosion Protection for Mg-1.0Ca Alloy
Received date: 2014-05-07
Revised date: 2014-05-19
Online published: 2014-05-25
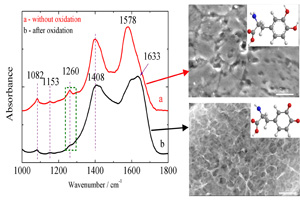
关键词: 氧化处理; 镁合金; 贻贝吸附蛋白(Mefp-1); 电化学阻抗谱
侯瑞青 , 蒋平丽 , 董士刚 , 林昌健 . 镁钙合金表面贻贝类吸附蛋白膜的NaIO4氧化处理及抗腐蚀性能[J]. 电化学, 2015 , 21(1) : 58 -65 . DOI: 10.13208/j.electrochem.140507

Key words: Oxidation; Mg Alloy; Mussel Adhesive Protein (Mefp-1); EIS
/
| 〈 |
|
〉 |