

“金标银染”放大技术的羟基自由基灵敏检测
收稿日期: 2014-08-04
修回日期: 2014-09-25
网络出版日期: 2014-09-30
基金资助
国家自然科学基金项目(No. 21275041, No. 21235002, No. 21221003)资助
Sensitive Detection of Hydroxyl Radical based on Silver-Enhanced Gold Nanoparticle Label
Received date: 2014-08-04
Revised date: 2014-09-25
Online published: 2014-09-30
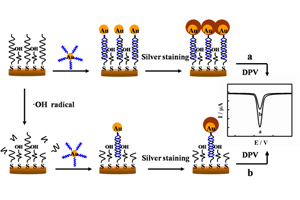
杨妍 , 喻鹏 , 张小华 , 陈金华 . “金标银染”放大技术的羟基自由基灵敏检测[J]. 电化学, 2015 , 21(1) : 22 -28 . DOI: 10.13208/j.electrochem.140442

/
| 〈 |
|
〉 |