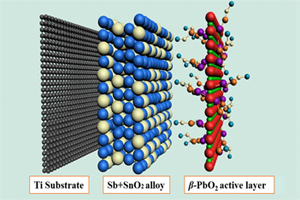
采用刷涂热解和电镀制得了β-PbO2/Sb-SnO2/Ti电极. 采用X射线衍射(X-ray Diffraction,XRD)和扫描电镜(Scanning Electron Microscope,SEM)进行分析与观察,Sb-SnO2中间层抑制PbF2的生成,NaF促进二氧化铅晶粒的成型与分散,消除了β-PbO2聚团. 据谢乐公式(Scherrer)计算晶粒尺寸为25.2 nm,电极表面结晶度高达100%. 极化测试显示,β-PbO2/Sb-SnO2/Ti电极扩散段电位区、析氧电位和Tafel斜率分别为1.85 ~ 2.15 V、2.08 V和0.84,优于β-PbO2/Ti电极的1.40 ~ 1.80 V、1.75 V和0.36. 使用β-PbO2/Sb-SnO2/Ti、β-PbO2/Ti电极在9 mA·cm-2电流密度对苯酚模拟废水处理240 min,前者COD(Chemical Oxygen Demand)去除率、电流效率(Instant Current Efficiency)高达90.1%和63.28%,优于后者66.9%和44.96%. 寿命测试表明,β-PbO2/Sb-SnO2/Ti电极与β-PbO2/Ti电极相比延长10倍,工业寿命可达8.6a,有较高的工程应用价值.
The combination technology of brush pyrolysis and electroplating was employed in the preparation of β-PbO2/Sb-SnO2/Ti electrode. X-ray Diffraction (XRD) and Scanning Electron Microscope (SEM) results showed that the Sb-SnO2 as an interlayer would restrain the formation of lead fluoride and the crystallization degree on the electrode surface could be as high as 100%. The grain size was calculated by Scherrer formula to be 25.2 nm and the agglomeration of lead dioxide was effectively eliminated. The potential span of diffusion control phase, oxygen evolution potential, Tafel slope for the β-PbO2/Sb-SnO2/Ti electrode during the polarization were 1.85 ~ 2.15 V, 2.08 V and 0.84, respectively, which exhibited much better electrochemical properties as compared with those of the β-PbO2/Ti electrode with the values of 1.40 ~ 1.80 V, 1.75 V and 0.36, respectively. Furthermore, the β-PbO2/Sb-SnO2/Ti and β-PbO2/Ti electrodes were taken as anodes participating in the electrocatalysis degradation of phenol simulated wastewater under current density 9 mA·cm-2 within 240 min. Experimental results revealed that the efficiencies of chemical oxygen demand (COD) removal and instant current efficiency (ICE) during phenol degradation were 90.1% and 63.28% for the β-PbO2/Sb-SnO2/Ti electrode, while 66.9% and 44.96% for the β-PbO2/Ti electrode. Ultimately, the accelerated life test was performed to evaluate the service time of β-PbO2/Sb-SnO2/Ti. The results presented that the industrial life of β-PbO2/Sb-SnO2/Ti was 8.6a, which is 10 times longer than that of β-PbO2/Ti,suggesting that β-PbO2/Sb-SnO2/Ti would have a relatively high engineering application value.