

Pt/DNA-MWCNTs/GC电极制备及其H2O2还原的电催化活性
收稿日期: 2013-12-24
修回日期: 2014-04-15
网络出版日期: 2014-04-20
基金资助
国家自然科学基金项目(No. 21263002)、广西自然科学基金项目(No. 2013GXNSFAA019024)、广西教育厅科学技术研究项目(No. 2013YB026)、药用资源化学与药物分子工程教育部重点实验室课题(No. CMEMR2012-A3)和广西研究生教育创新计划(No. 2010106020703M67)资助
Preparation of Pt/DNA-MWCNTs/GC Electrode and Its Electrocatalytic Activity toward H2O2 Reduction
Received date: 2013-12-24
Revised date: 2014-04-15
Online published: 2014-04-20
范丽丽 , 武丽娜 , 屈志宇 , 刘丹凤 , 张俊明 , 樊思明 , 樊友军 . Pt/DNA-MWCNTs/GC电极制备及其H2O2还原的电催化活性[J]. 电化学, 2014 , 20(5) : 459 -464 . DOI: 10.13208/j.electrochem.131165
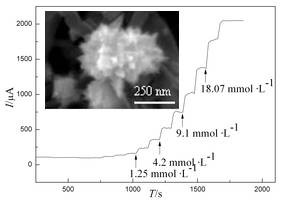
Key words: DNA; functionalization; multi-walled carbon nanotubes; Pt; H2O2 detection
/
| 〈 |
|
〉 |