

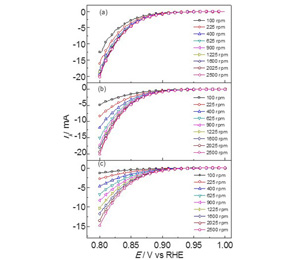
利用K-L方程估算旋转圆盘电极体系反应动力学电流的误差来源分析
收稿日期: 2014-01-15
修回日期: 2014-05-27
网络出版日期: 2014-06-03
基金资助
国家自然科学基金项目(No. 21073176)及科技部973计划项目(No. 2010CB923302)资助
On the Origin of the Errors of ik as Estimated from K-L Equation in Rotating Disk Electrode System
Received date: 2014-01-15
Revised date: 2014-05-27
Online published: 2014-06-03
陈微 , 廖玲文 , 何政达 , 陈艳霞 . 利用K-L方程估算旋转圆盘电极体系反应动力学电流的误差来源分析[J]. 电化学, 2014 , 20(5) : 444 -451 . DOI: 10.13208/j.electrochem.131167

/
| 〈 |
|
〉 |