

基于碳纳米管的高灵敏度免标记电化学核酸适体传感电极
收稿日期: 2013-11-26
修回日期: 2014-04-09
网络出版日期: 2014-04-15
基金资助
国家自然科学基金项目(No. 21005090)、中国博士后基金项目(No. 2012M510136)和中国博士基金特别资助项目(No. 2013T60774)
A Sensitive and Label-Free Electrochemical Aptasensor based on Signal Amplification of Carbon Nanotubes
Received date: 2013-11-26
Revised date: 2014-04-09
Online published: 2014-04-15
邓春艳 , 范慧敏 , 向娟 , 李元建 . 基于碳纳米管的高灵敏度免标记电化学核酸适体传感电极[J]. 电化学, 2014 , 20(4) : 386 -391 . DOI: 10.13208/j.electrochem.131161
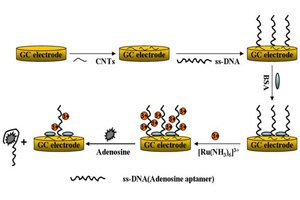
Key words: carbon nanotubes; aptamer; label-free; adenosine
/
| 〈 |
|
〉 |