

绿脓杆菌阳极的微生物燃料电池含溶解氧性能研究
收稿日期: 2013-11-26
修回日期: 2014-02-26
网络出版日期: 2014-03-04
基金资助
国家自然科学基金委员会与英国皇家学会合作交流项目(No. 21311130120)资助
Effecting of Dissolved Oxygen on Microbial Fuel Cells Based on Pseudomonas Aeruginosa
Received date: 2013-11-26
Revised date: 2014-02-26
Online published: 2014-03-04
曹昌丽 , 陈立香 , 吴冉冉 , 张耿崚 , 赵峰 . 绿脓杆菌阳极的微生物燃料电池含溶解氧性能研究[J]. 电化学, 2014 , 20(4) : 382 -385 . DOI: 10.13208/j.electrochem.131162
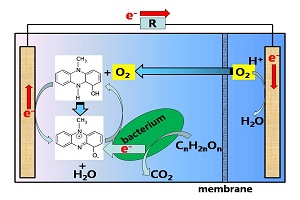
Key words: microbial fuel cells; Pseudomonas aeruginosa; anode; oxygen; pyocyanine
/
| 〈 |
|
〉 |