

Pt-TiO2/Graphene催化剂的氧还原和甲醇氧化电催化性能研究
收稿日期: 2014-01-17
修回日期: 2014-03-10
网络出版日期: 2014-03-20
基金资助
国家自然科学基金项目(No. 21176111)、浙江省重大科技专项(No. 2012C11014)和绍兴县科技攻关计划项目(No. 2012506)资助
Preparation of Pt-TiO2/Graphene Composites with High Catalytic Activity towards Methanol Oxidation and Oxygen Reduction Reaction
Received date: 2014-01-17
Revised date: 2014-03-10
Online published: 2014-03-20
戚利 , 殷瑛 , 涂文广 , 吴兵兵 , 王兆生 , 刘建国 , 顾军 , 邹志刚 . Pt-TiO2/Graphene催化剂的氧还原和甲醇氧化电催化性能研究[J]. 电化学, 2014 , 20(4) : 377 -381 . DOI: 10.13208/j.electrochem.131171
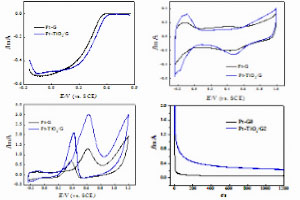
Key words: TiO2; graphene; ORR; methanol oxidation
/
| 〈 |
|
〉 |